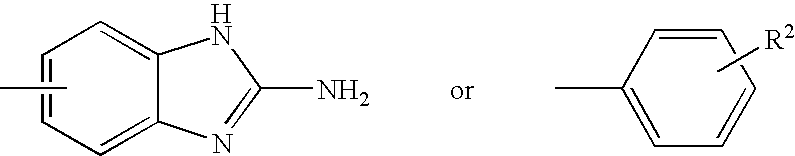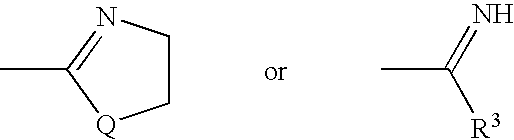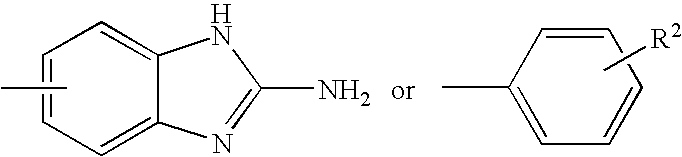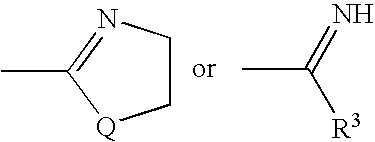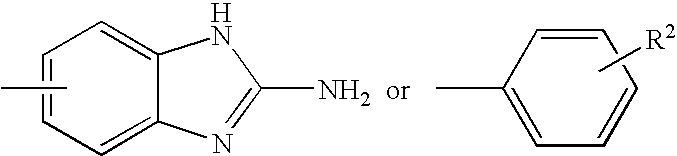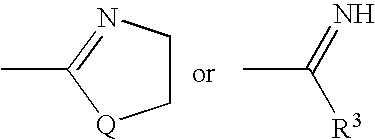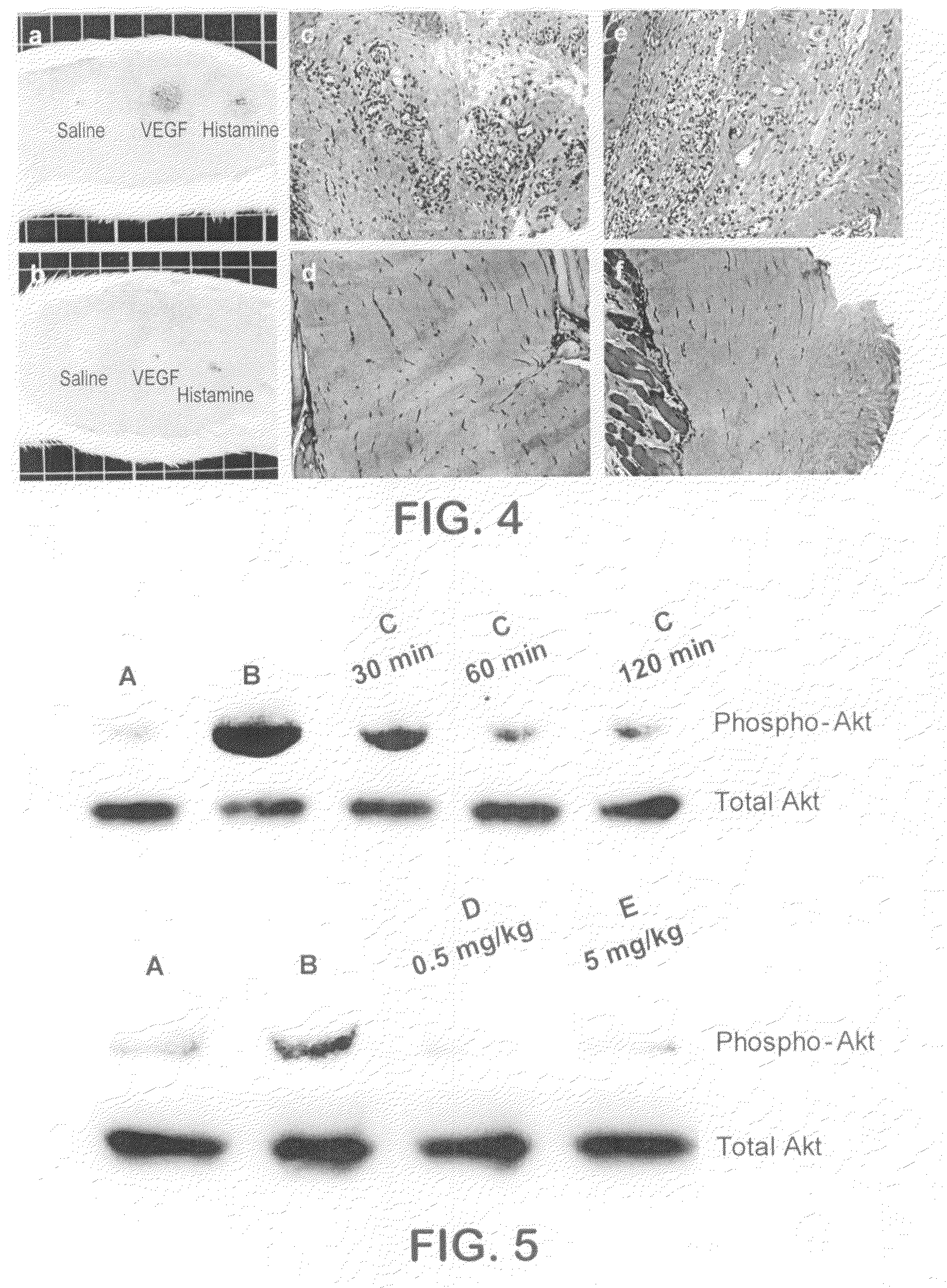Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
76 results about "EDEMA RETINAL" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Macular edema occurs when fluid and protein deposits collect on or under the macula of the eye (a yellow central area of the retina) and causes it to thicken and swell (edema).
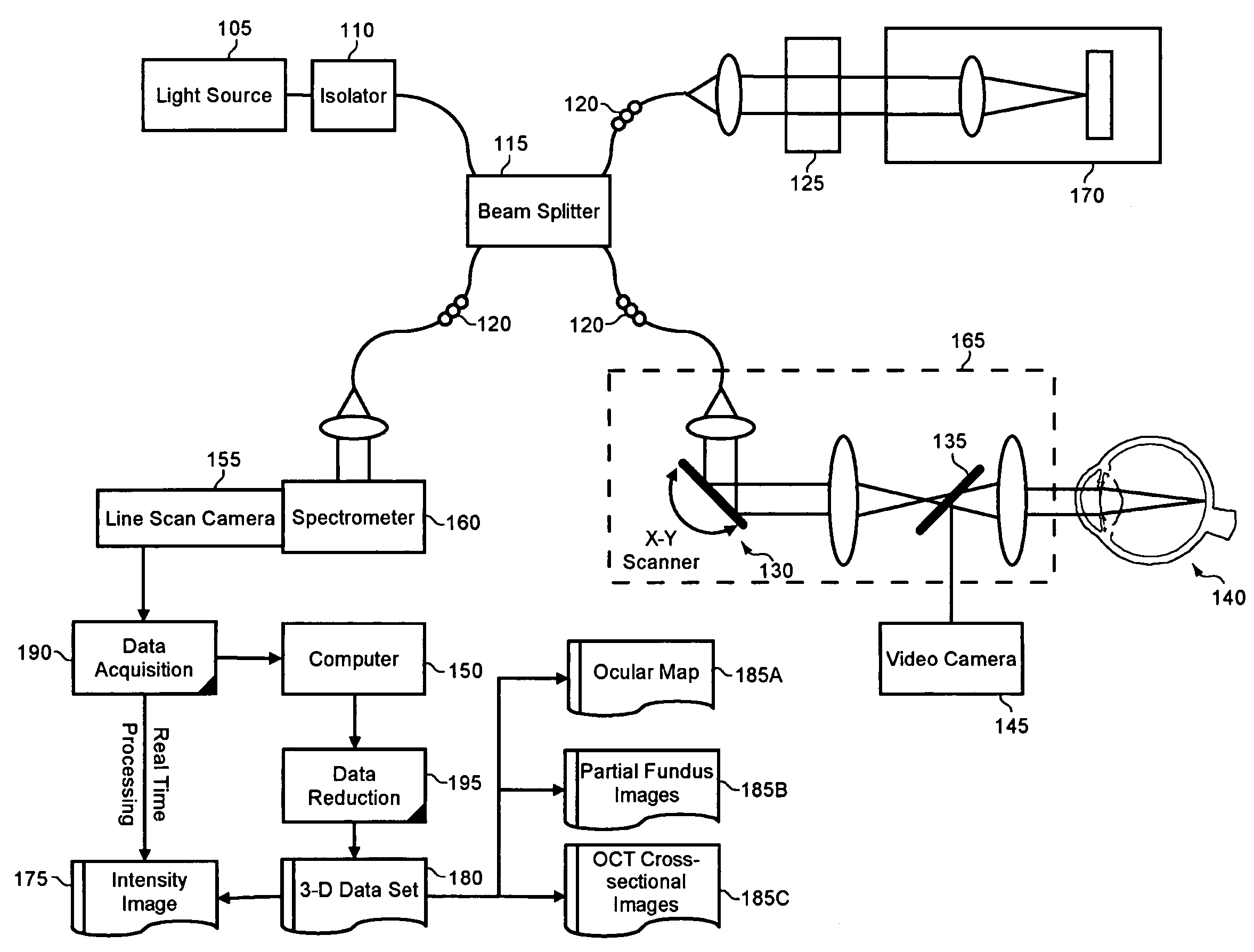
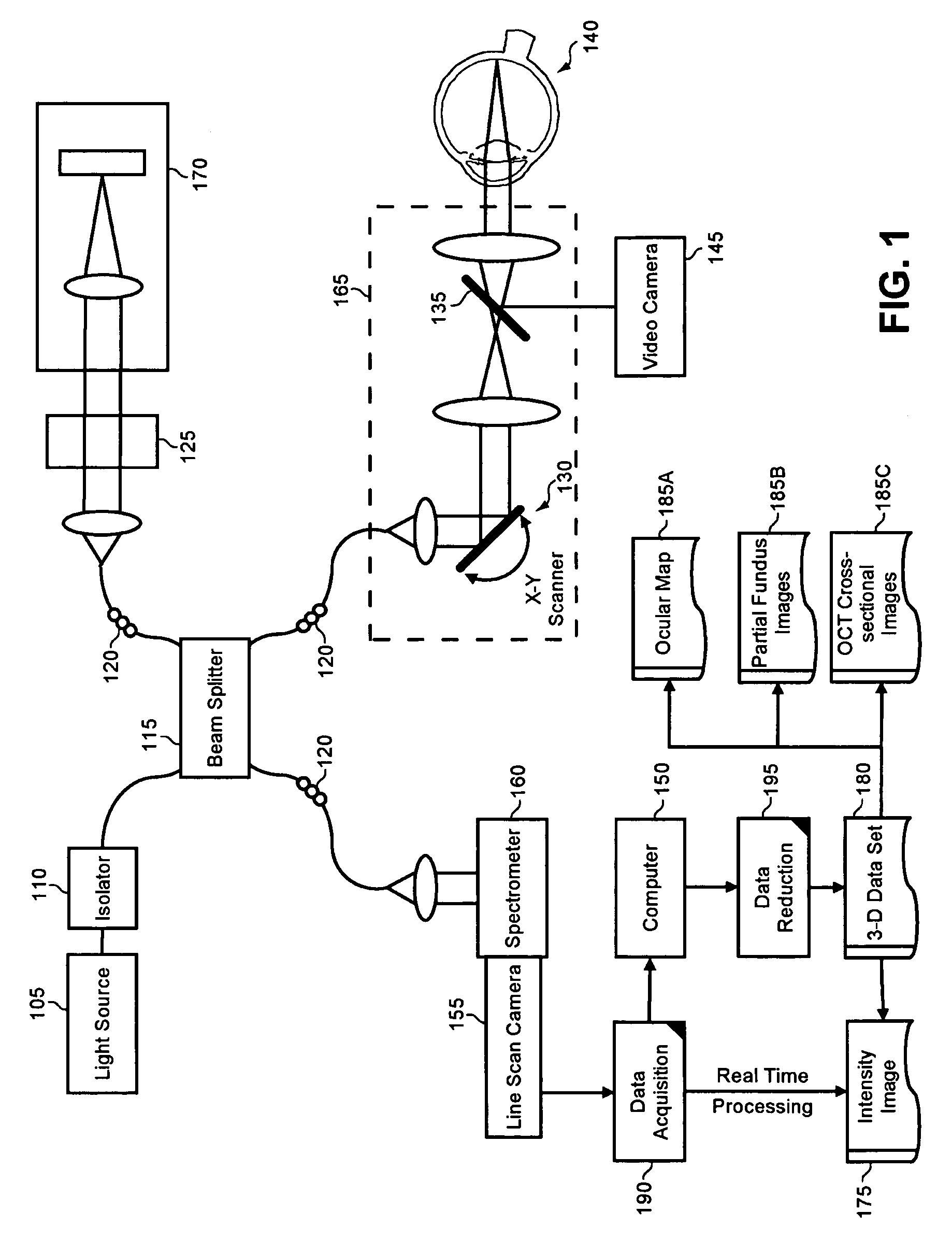
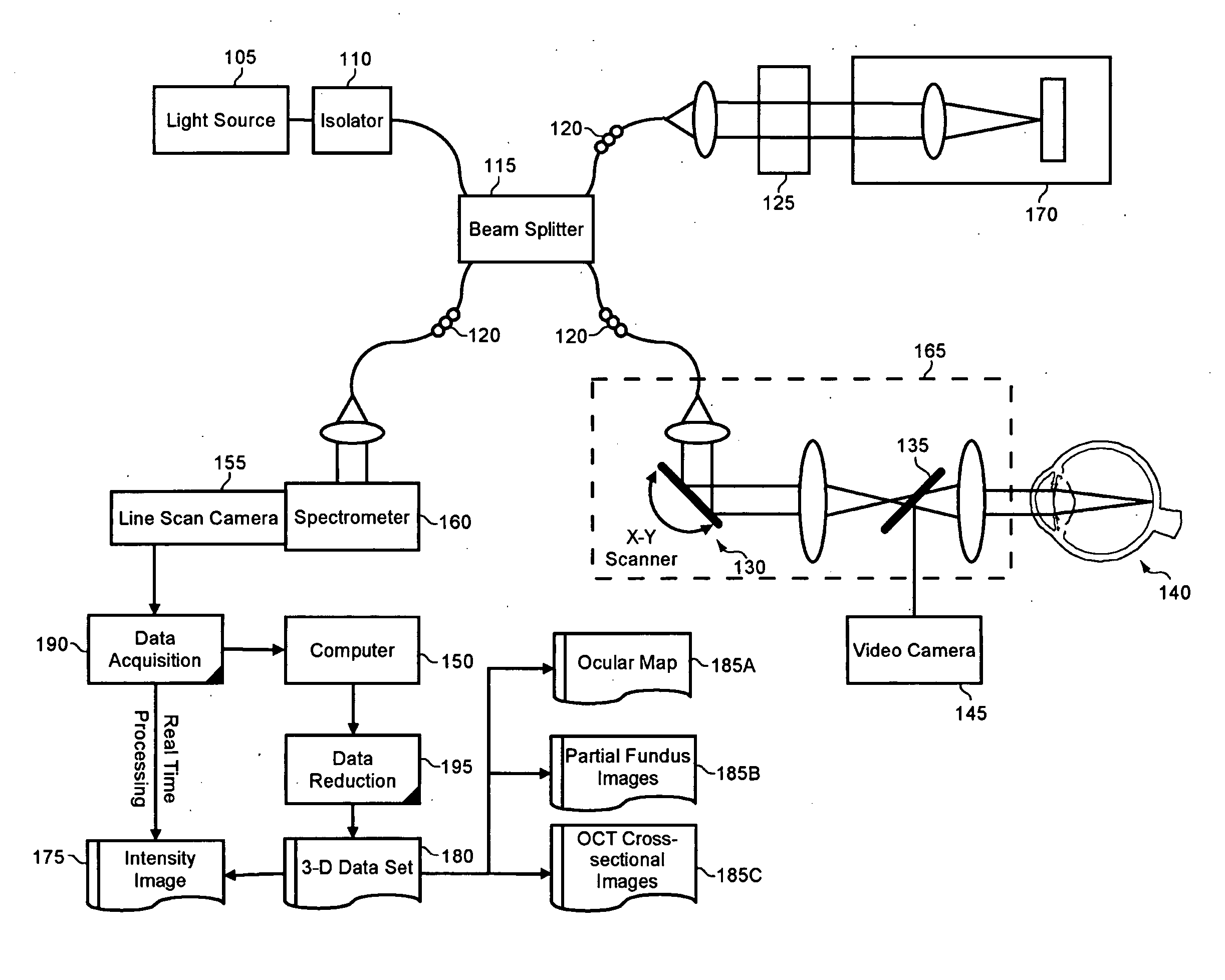
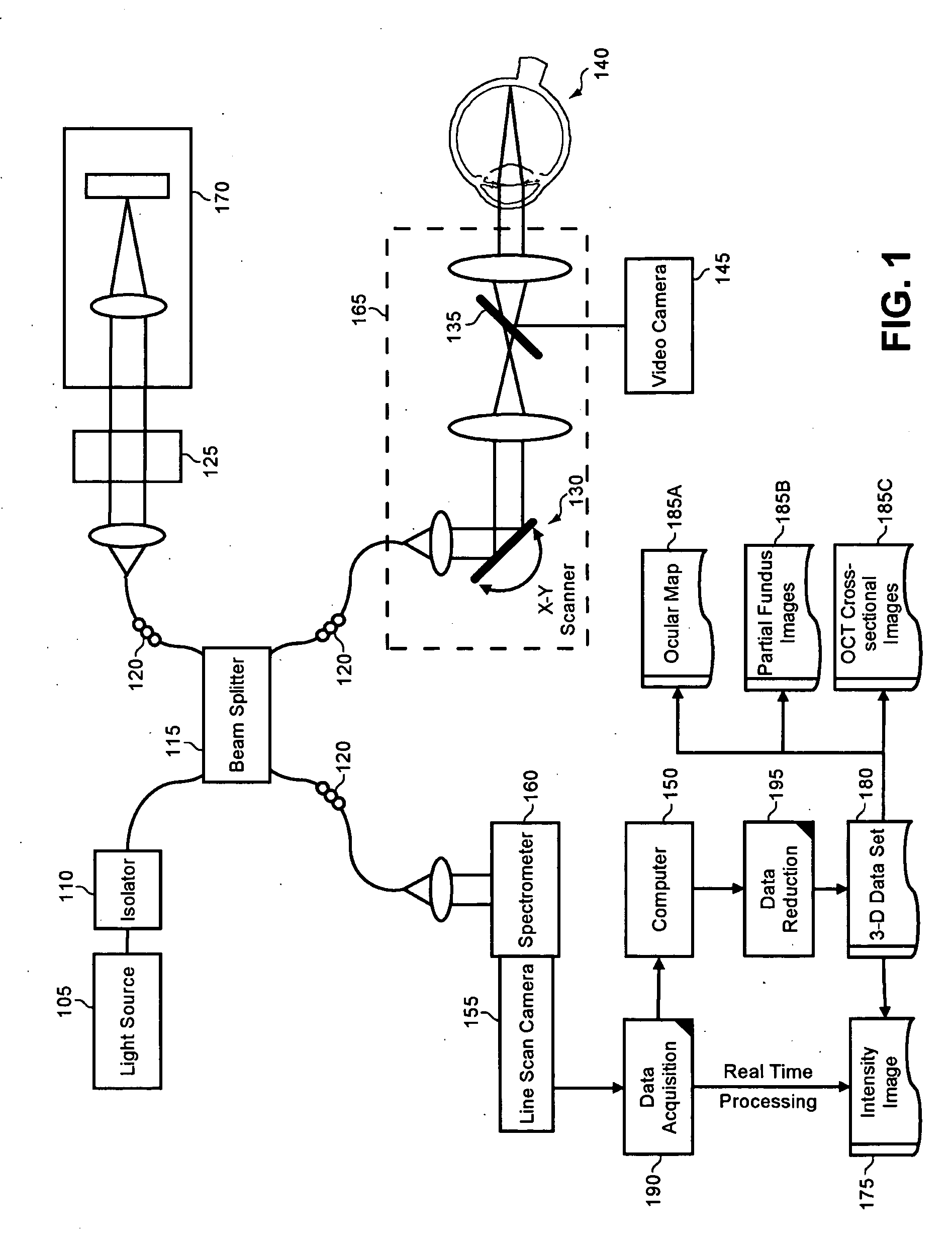
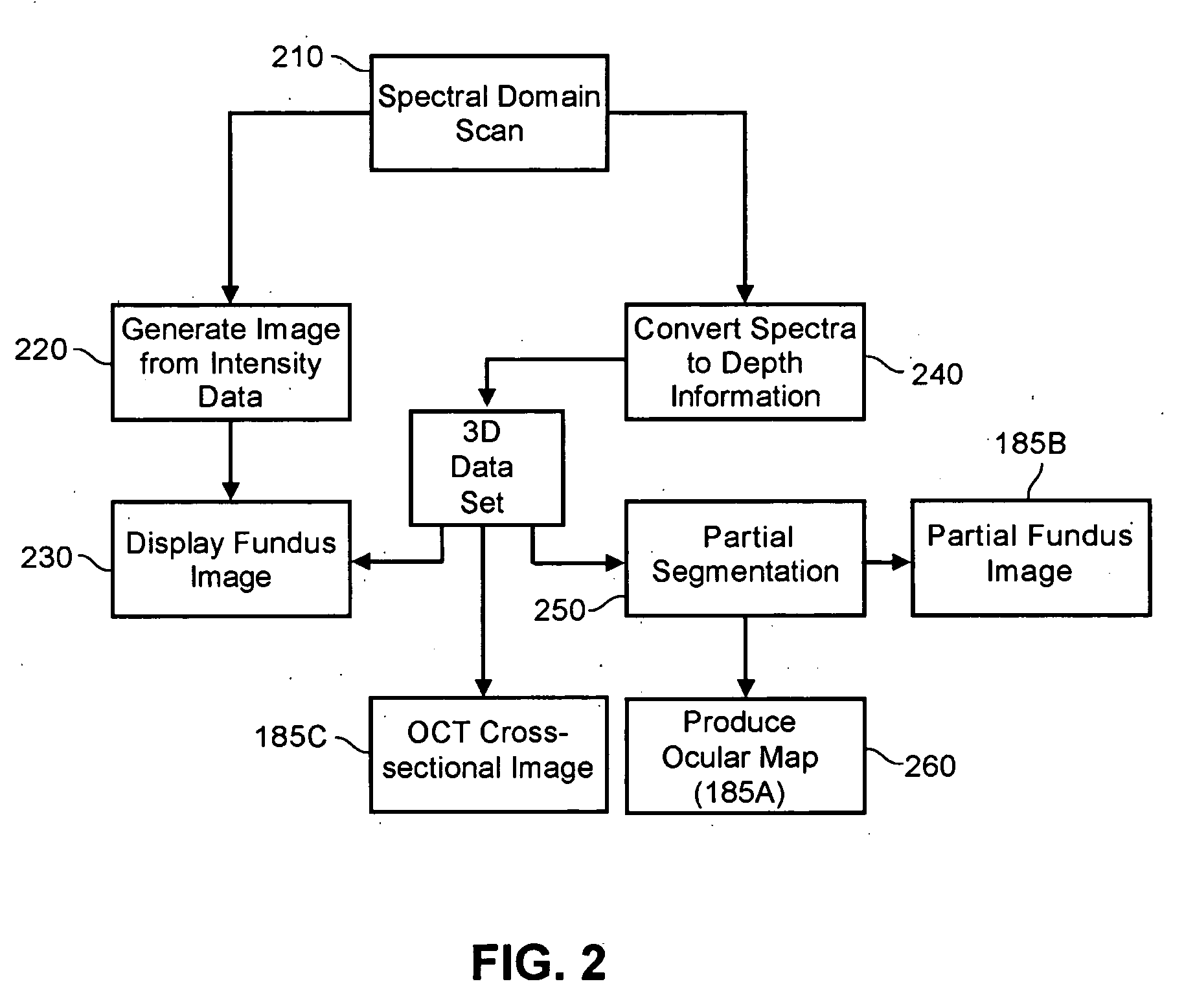
Enhanced optical coherence tomography for anatomical mapping
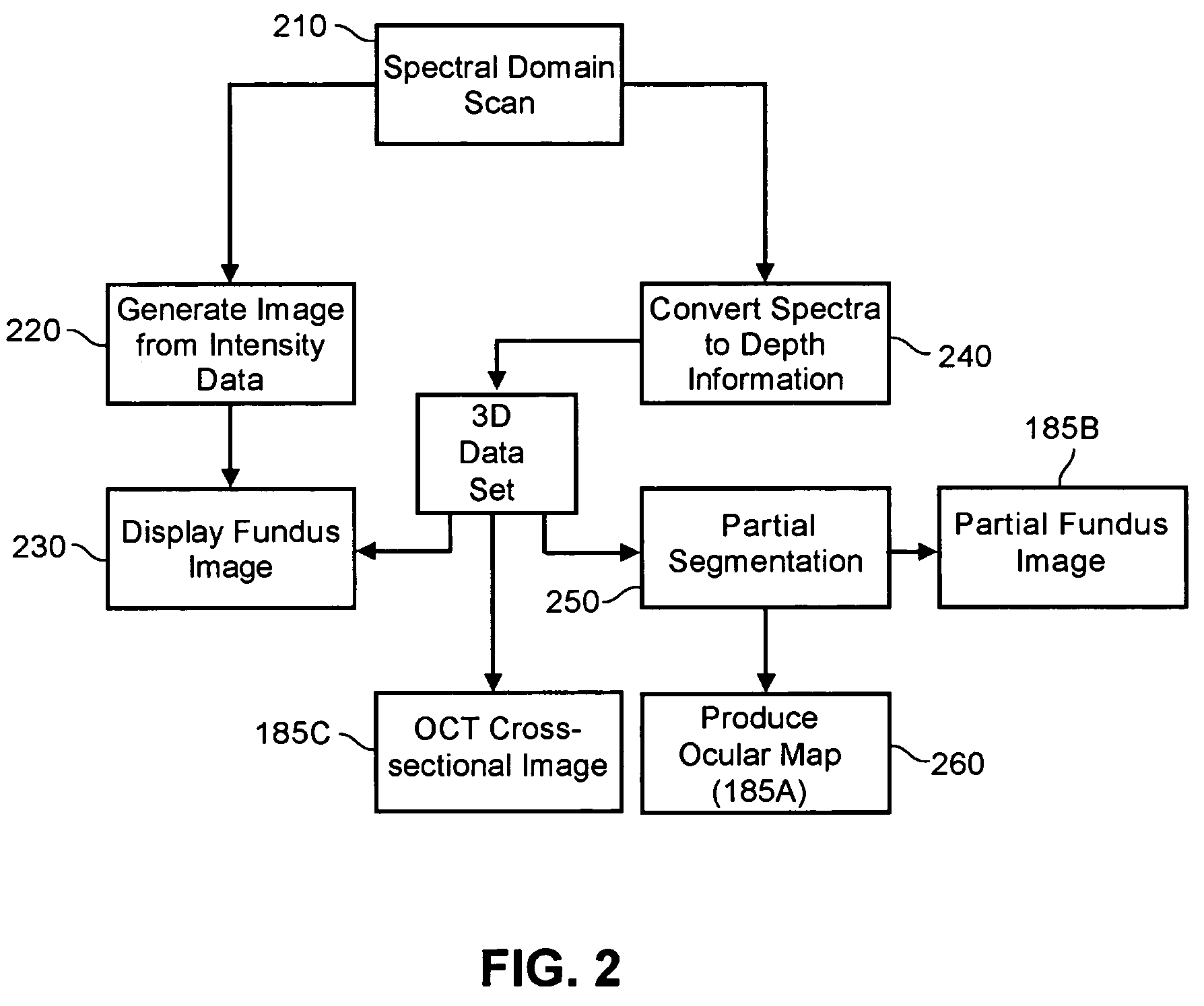
A system, method and apparatus for anatomical mapping utilizing optical coherence tomography. In the present invention, 3-dimensional fundus intensity imagery can be acquired from a scanning of light back-reflected from an eye. The scanning can include spectral domain scanning, as an example. A fundus intensity image can be acquired in real-time. The 3-dimensional data set can be reduced to generate an anatomical mapping, such as an edema mapping and a thickness mapping. Optionally, a partial fundus intensity image can be produced from the scanning of the eye to generate an en face view of the retinal structure of the eye without first requiring a full segmentation of the 3-D data set. Advantageously, the system, method and apparatus of the present invention can provide quantitative three-dimensional information about the spatial location and extent of macular edema and other pathologies. This three-dimensional information can be used to determine the need for treatment, monitor the effectiveness of treatment and identify the return of fluid that may signal the need for re-treatment.
Owner:UNIV OF MIAMI
Enhanced optical coherence tomography for anatomical mapping
ActiveUS20060119858A1Enhanced anatomical mappingReduction in total information contentEye diagnosticsUsing optical meansData setSpectral domain
A system, method and apparatus for anatomical mapping utilizing optical coherence tomography. In the present invention, 3-dimensional fundus intensity imagery can be acquired from a scanning of light back-reflected from an eye. The scanning can include spectral domain scanning, as an example. A fundus intensity image can be acquired in real-time. The 3-dimensional data set can be reduced to generate an anatomical mapping, such as an edema mapping and a thickness mapping. Optionally, a partial fundus intensity image can be produced from the scanning of the eye to generate an en face view of the retinal structure of the eye without first requiring a full segmentation of the 3-D data set. Advantageously, the system, method and apparatus of the present invention can provide quantitative three-dimensional information about the spatial location and extent of macular edema and other pathologies. This three-dimensional information can be used to determine the need for treatment, monitor the effectiveness of treatment and identify the return of fluid that may signal the need for re-treatment.
Owner:UNIV OF MIAMI
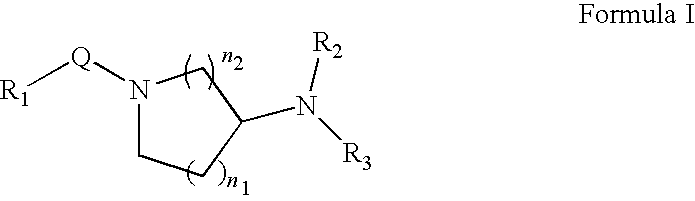
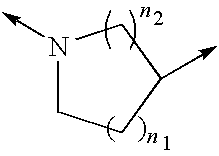
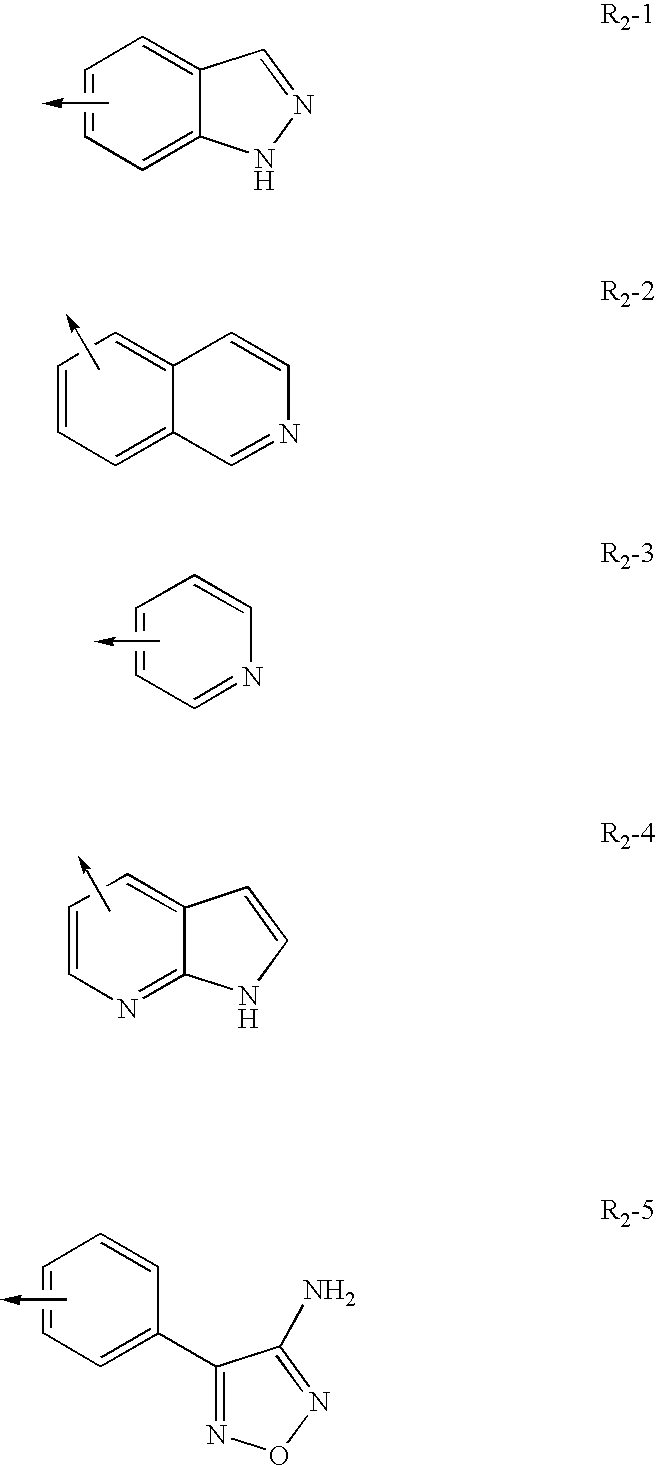
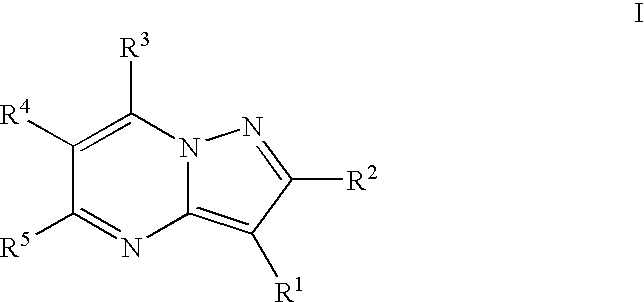
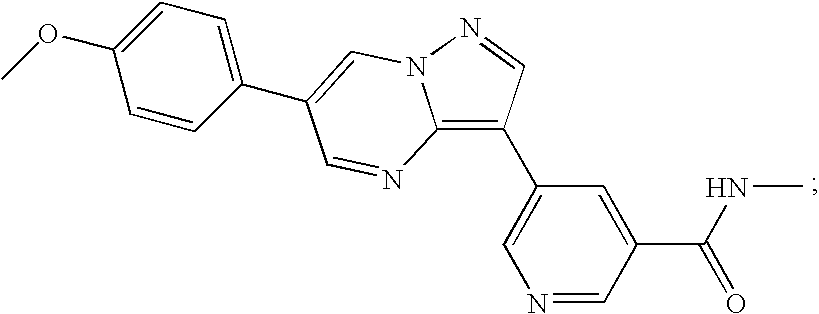
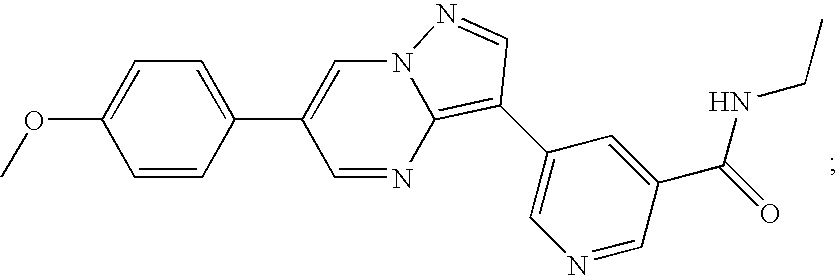
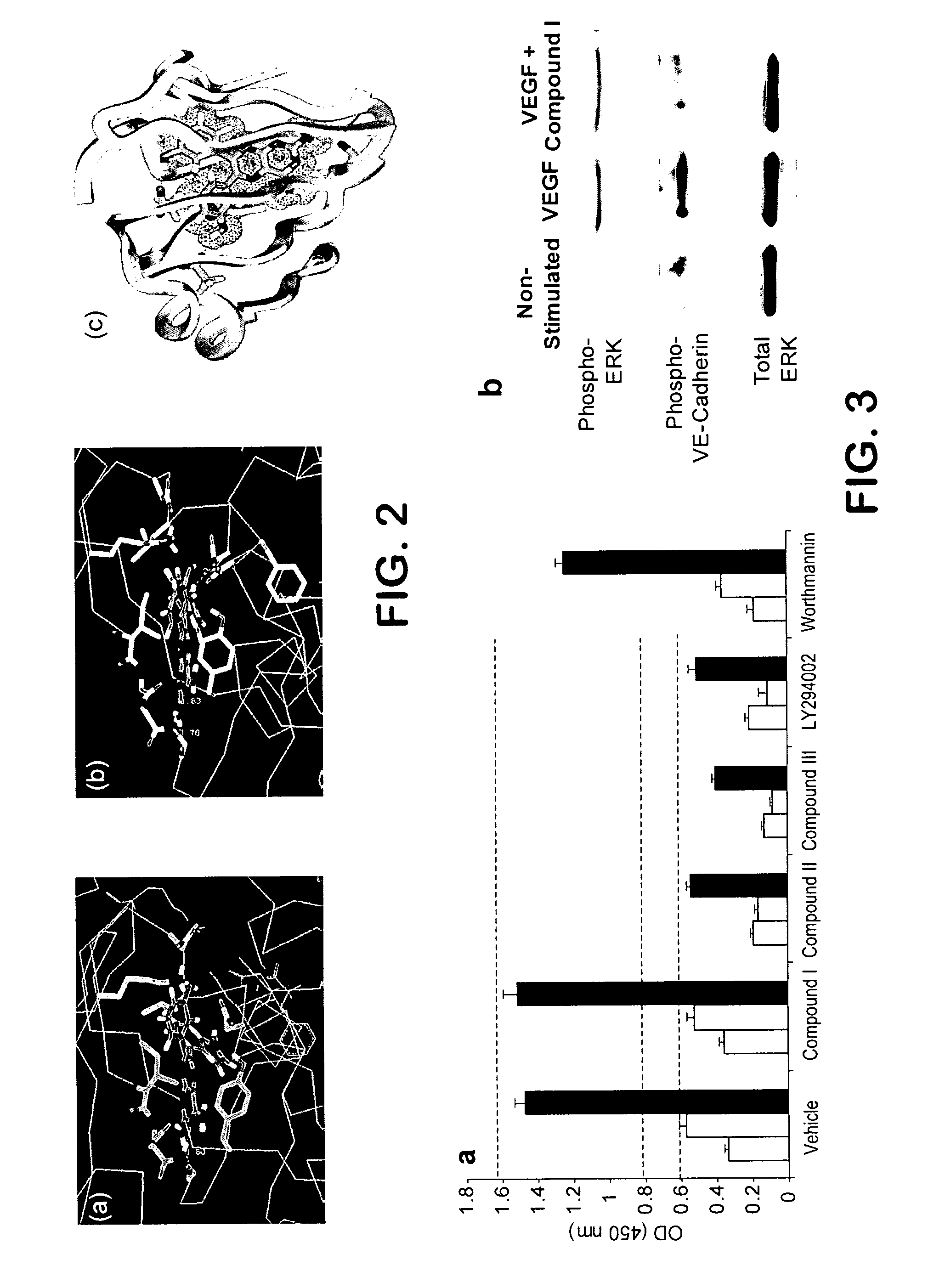
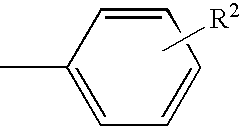
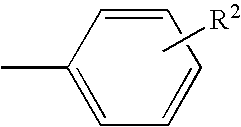
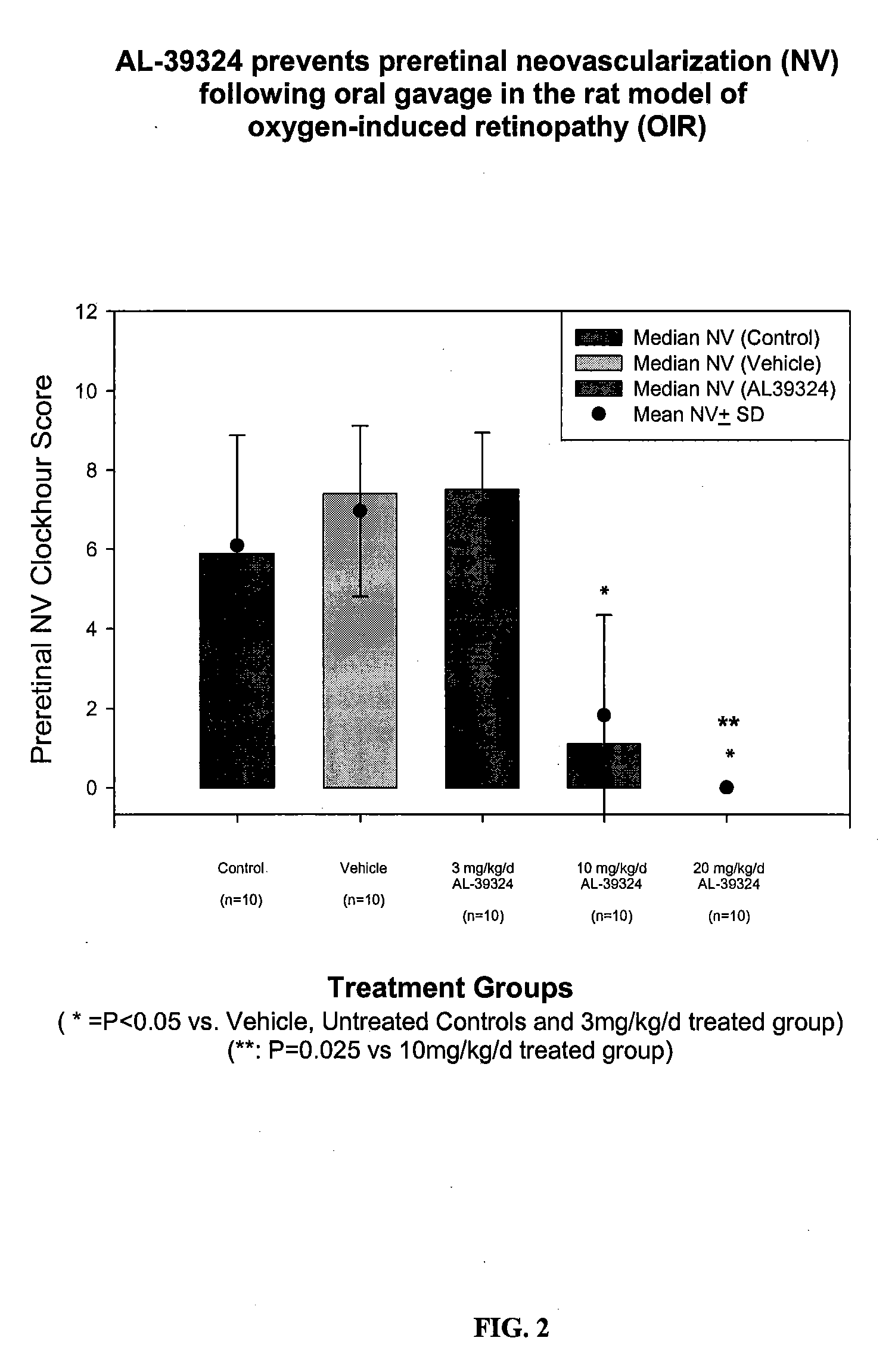
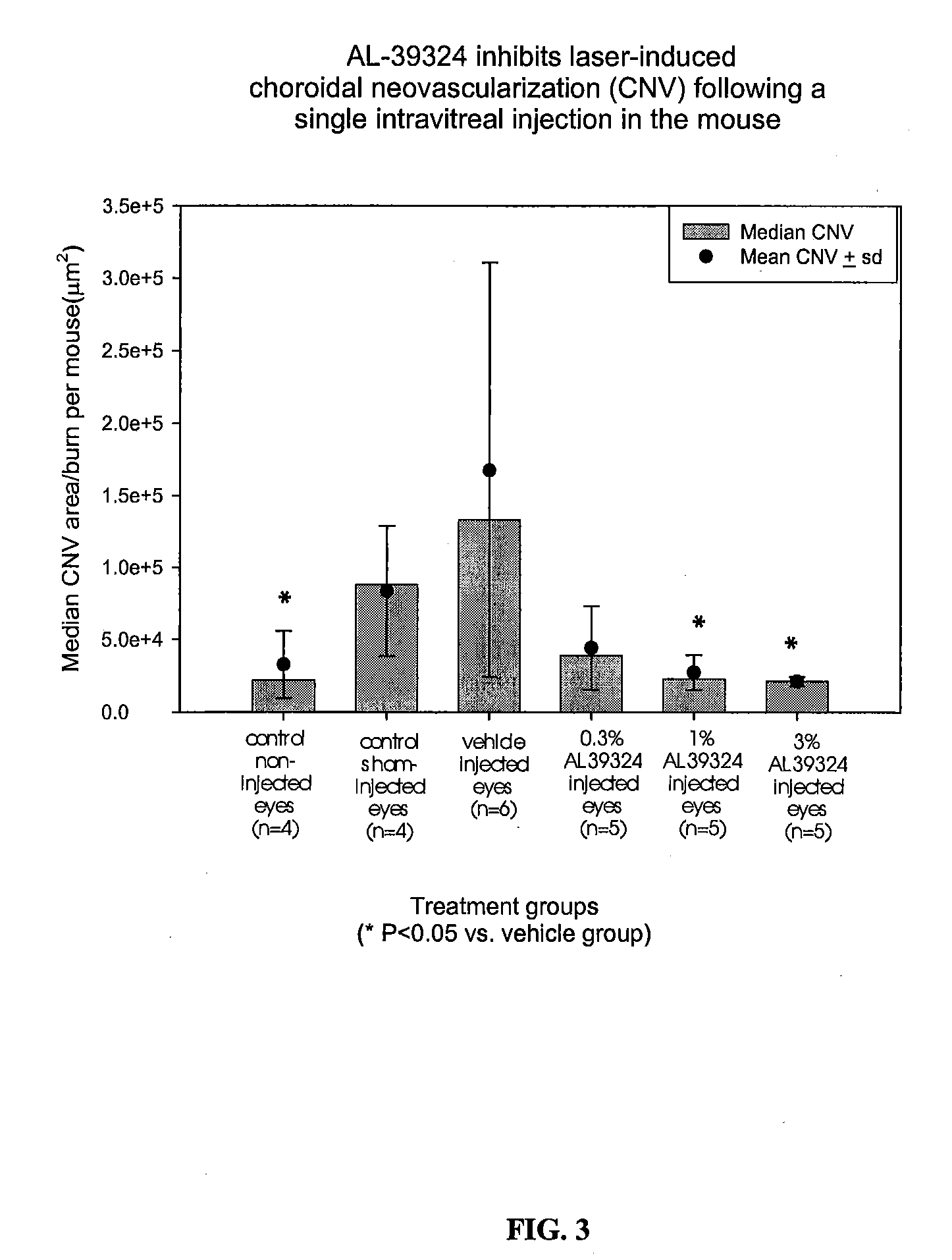
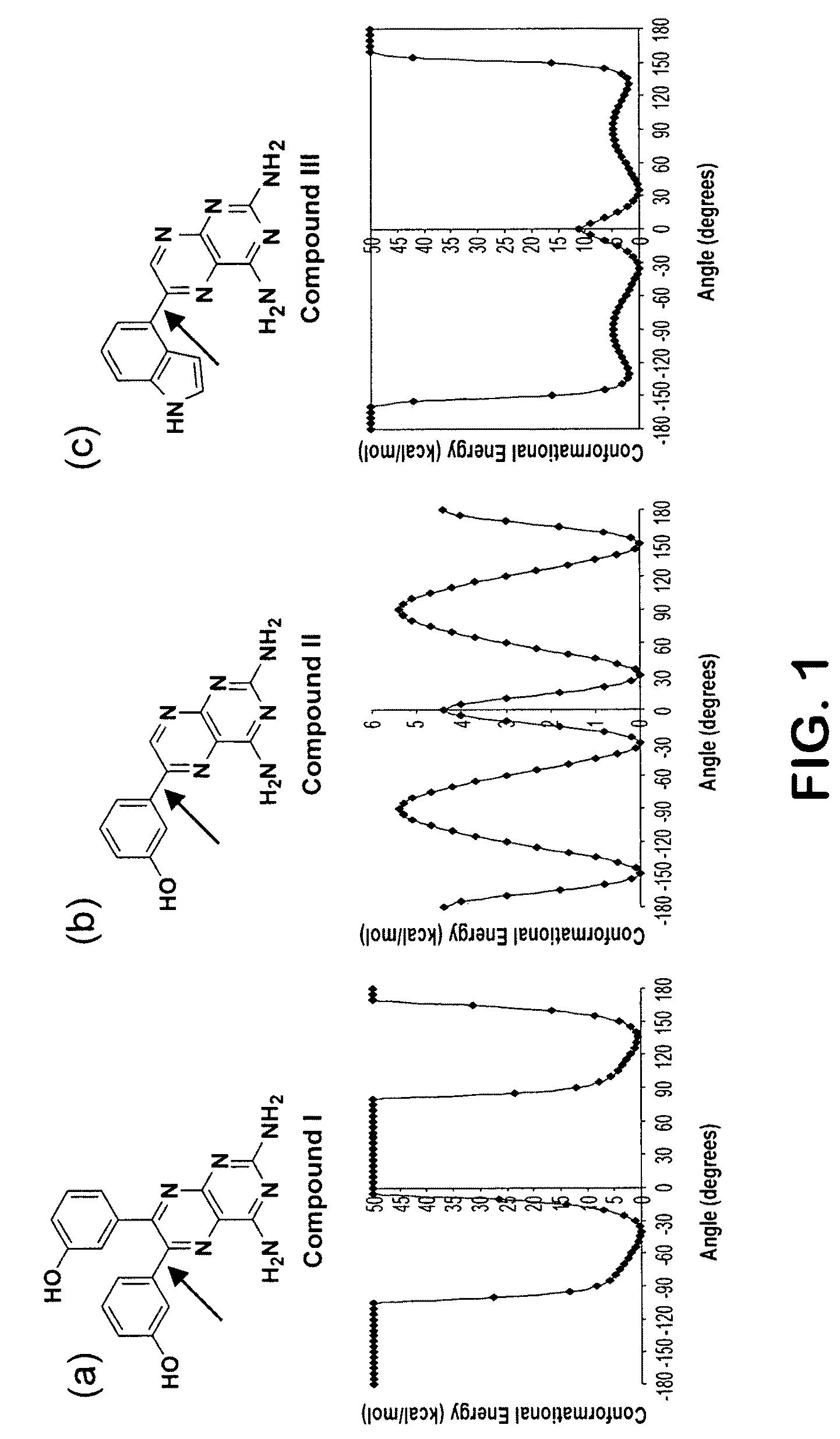
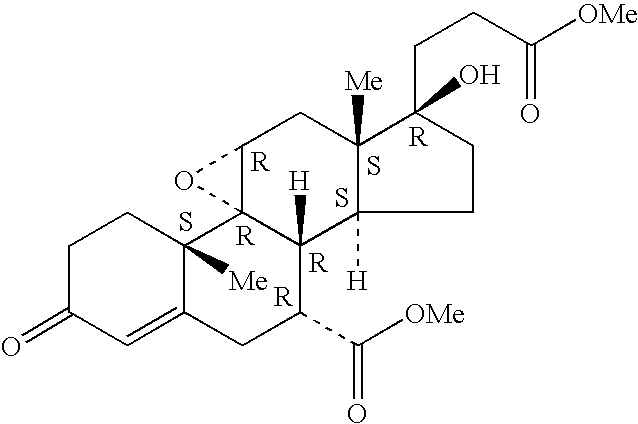
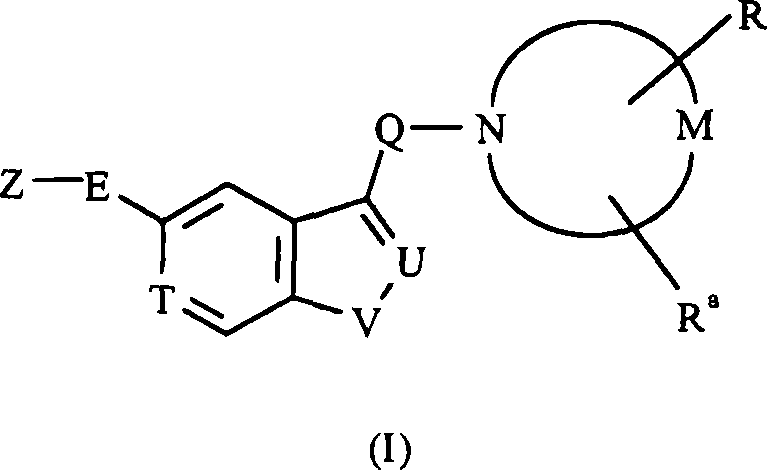
Substituted pyrazolo[1,5-A]pyrimidines as tyrosine kinase inhibitors
The present invention relates to compounds which inhibit, regulate and / or modulate tyrosine kinase signal transduction, compositions which contain these compounds, and methods of using them to treat tyrosine kinase-dependent diseases and conditions, such as angiogenesis, cancer, tumor growth, atherosclerosis, age related macular degeneration, diabetic retinopathy, macular edema, retinal ischemia, inflammatory diseases, and the like in mammals.
Owner:MERCK SHARP & DOHME CORP
Methods and compositions for treating eye disorders
InactiveUS20040234532A1Efficiently provideAntibacterial agentsBacterial antigen ingredientsUveitisViral Conjunctivitis
The present invention provides methods of treating an eye disorder. The methods comprise a step of locally administering a Clostridial toxin to the eye of a patient to treat the disorder. The eye disorder may be associated with an inflammation of the eye, including for example, bacterial conjunctivitis, fungal conjunctivitis, viral conjunctivitis, uveitis, keratic precipitates, macular edema, and inflammation response after intra-ocular lens implantation. The Clostridial toxin may be produced by a Clostridial beratti, a Clostridia butyricum, a Clostridial tetani bacterium and / or a Clostridial botulinum.
Owner:ALLERGAN INC
Method for treating ophthalmic diseases using rho kinase inhibitor compounds
This invention is directed to methods of preventing or treating ocular diseases with inflammation, excessive cell proliferation, remodeling, neurite retraction, corneal neurodegeneration, excessive vaso-permeability and edema. Particularly, this invention relates to methods treating ocular diseases such as allergic conjunctivitis, corneal hyposensitivity, neurotrophic keratopathy, dry eye disease, proliferative vitreal retinopathy, macular edema, macular degeneration, and blepharitis, using novel Rho kinase inhibitor compounds. The method comprises identifying a subject in need of the treatment, and administering to the subject an effective amount of a novel Rho kinase inhibitor compound to treat the disease.
Owner:INSPIRE PHARMA
Mapping and diagnosis of macular edema by optical coherence tomography
The present invention discloses a method for comparing the detection of clinically significant diabetic macular edema by an optical coherence tomography (OCT) grid scanning protocol and biomicroscopic examination. Also provided are computer implemented, automated systems for performing the method thereof and computer readable media encoding the method thereof.
Owner:UNIV OF SOUTHERN CALIFORNIA
Compounds useful in inhibiting vascular leakage, inflammation and fibrosis and methods of making and using same
InactiveUS20050250694A1Prevent fibrosisInhibit angiogenesisPeptide/protein ingredientsDepsipeptidesPIGMENT EPITHELIUM-DERIVED FACTORAngiostatin
The present invention is directed to a method of inhibiting at least one of vascular leakage, inflammation and fibrosis in an animal by administering to the animal a vascular leakage inhibiting amount of a composition, wherein at a substantially higher amount the composition is effective in inhibiting angiogenesis, and wherein the anti-angiogenic activity of the composition is separate from the vascular leakage inhibiting activity of the composition. The animal experiencing at least one of vascular leakage, inflammation and fibrosis has a disease selected from the group consisting of diabetes, chronic inflammation, brain edema, arthritis, uvietis, macular edema, cancer, hyperglycemia, a kidney inflammatory disease, a disorder resulting in kidney fibrosis, a disorder of the kidney resulting in proteinuria, and combinations thereof. The composition capable of inhibiting at least one of vascular leakage, inflammation and fibrosis is selected from the group consisting of angiostatin, fragments of angiostatin, analogs or derivatives of angiostatin, kringle 5 of plasminogen, fragments of kringle 5 of plasminogen, analogs or derivatives of kringle 5 of plasminogen, pigment epithelium-derived factor, fragments of pigment epithelium-derived factor, analogs or derivatives of pigment epithelium-derived factor and combinations thereof.
Owner:THE BOARD OF RGT UNIV OF OKLAHOMA
Tyrosine kinase inhibitors
InactiveUS20060025426A1Organic active ingredientsBiocideDiabetic retinopathyTyrosine-kinase inhibitor
The present invention relates to compounds which inhibit, regulate and / or modulate tyrosine kinase signal transduction, compositions which contain these compounds, and methods of using them to treat tyrosine kinase-dependent diseases and conditions, such as angiogenesis, cancer, tumor growth, atherosclerosis, age related macular degeneration, diabetic retinopathy, macular edema, retinal ischemia, inflammatory diseases, and the like in mammals.
Owner:MERCK SHARP & DOHME CORP
Methods and compositions for treating eye disorders
Owner:ALLERGAN INC
Compositions and methods for treating ocular diseases
InactiveUS20150050277A1Dipeptide ingredientsNitro compound active ingredientsUveitisMacula lutea degeneration
Disclosed herein are compositions and methods for treating ocular diseases, inter alia, diabetic macular edema, age-related macular degeneration (wet form), choroidal neovascularization, diabetic retinopathy, retinal vein occlusion (central or branch), ocular trauma, surgery induced edema, surgery induced neovascularization, cystoid macular edema, ocular ischemia, uveitis, and the like. These diseases or conditions are characterized by changes in the ocular vasculature whether progressive or non-progressive, whether a result of an acute disease or condition, or a chronic disease or condition.
Owner:EYEPOINT PHARMA INC
Kinase inhibitors and methods of use thereof
InactiveUS20070259876A1Inhibiting and reducing vascular leakageReduce vascular leakageBiocideOrganic chemistryUveitisAutoimmune condition
Compositions and methods and are provided for treating disorders associated with compromised vasculostasis. Invention methods and compositions are useful for treating a variety of disorders including for example, stroke, myocardial infarction, cancer, ischemia / reperfusion injury, autoimmune diseases such as rheumatoid arthritis, eye diseases such as uveitis, retinopathies or macular degeneration, macular edema or other vitreoretinal diseases, inflammatory diseases such as autoimmune diseases, vascular leakage syndrome, edema, or diseases involving leukocyte activation, transplant rejection, respiratory diseases such as asthma, adult or acute respiratory distress syndrome (ARDS), chronic obstructive pulmonary disease, and the like.
Owner:TARGEGEN
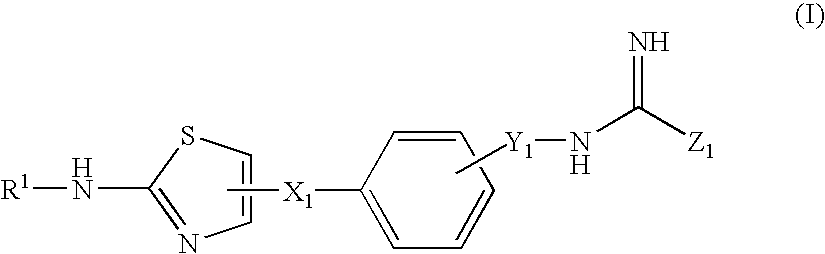
Thiazole derivatives
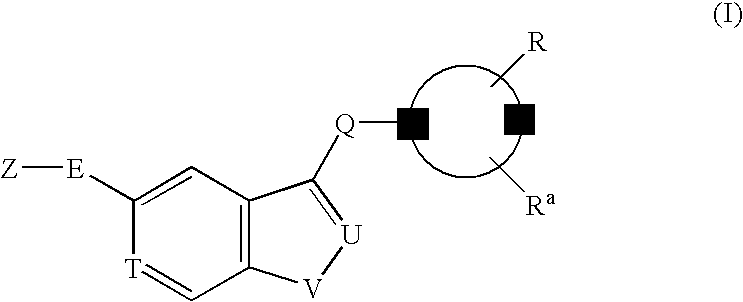
A compound of the formula (I): R<1>-NH-X-Y-Z (I) wherein each symbol is as defined in the specification, or a pharmaceutically acceptable salt thereof useful as a vascular adhesion protein-1 (VAP-1) inhibitor, a pharmaceutical composition, a method for preventing or treating a VAP-1 associated disease, especially macular edema, which method includes administering an effective amount of the compound or a pharmaceutically acceptable salt thereof to a mammal, and the like.
Owner:R TECH UENO
Method for treating vascular hyperpermeable disease
InactiveUS20060229346A1Useful in treatmentBiocideOrganic active ingredientsVASCULAR ADHESION PROTEIN 1Disease cause
The present invention provides a method for treating a vascular hyperpermeable disease (except macular edema), which method comprises administering to a patient in need thereof a vascular adhesion protein-1 (VAP-1) inhibitor in an amount sufficient to treat said patient for said disease. The agents are 2-acylamino thiazole compounds.
Owner:ASTELLAS PHARMA INC +1
Thiazole derivatives
A compound of the formula (I): R1—NH—X—Y-Z (I) wherein each symbol is as defined in the specification, or a pharmaceutically acceptable salt thereof useful as a vascular adhesion protein-1 (VAP-1) inhibitor, a pharmaceutical composition, a method for preventing or treating a VAP-1 associated disease, especially macular edema, which method includes administering an effective amount of the compound or a pharmaceutically acceptable salt thereof to a mammal, and the like.
Owner:ASTELLAS PHARMA INC
Fluorinated integrin antagonists
The present invention relates to fluorinated compounds of formula I and methods of synthesizing these compounds. The present invention also relates to pharmaceutical compositions containing the fluorinated compounds of the invention, and methods of treating macular degeneration, diabetic retinopathy (DR), macular edema, diabetic macular edema (DME), and macular edema following retinal vein occlusion (RVO), by administering these compounds and pharmaceutical compositions to subjects in need thereof.
Owner:OCUTERRA THERAPEUTICS INC
Diagnostic method and kit for implantation of a sustained release drug-delivery implant with a steroid
InactiveUS20050226814A1Minimally invasiveReduce swellingCompounds screening/testingOrganic active ingredientsSustained release drugMedicine
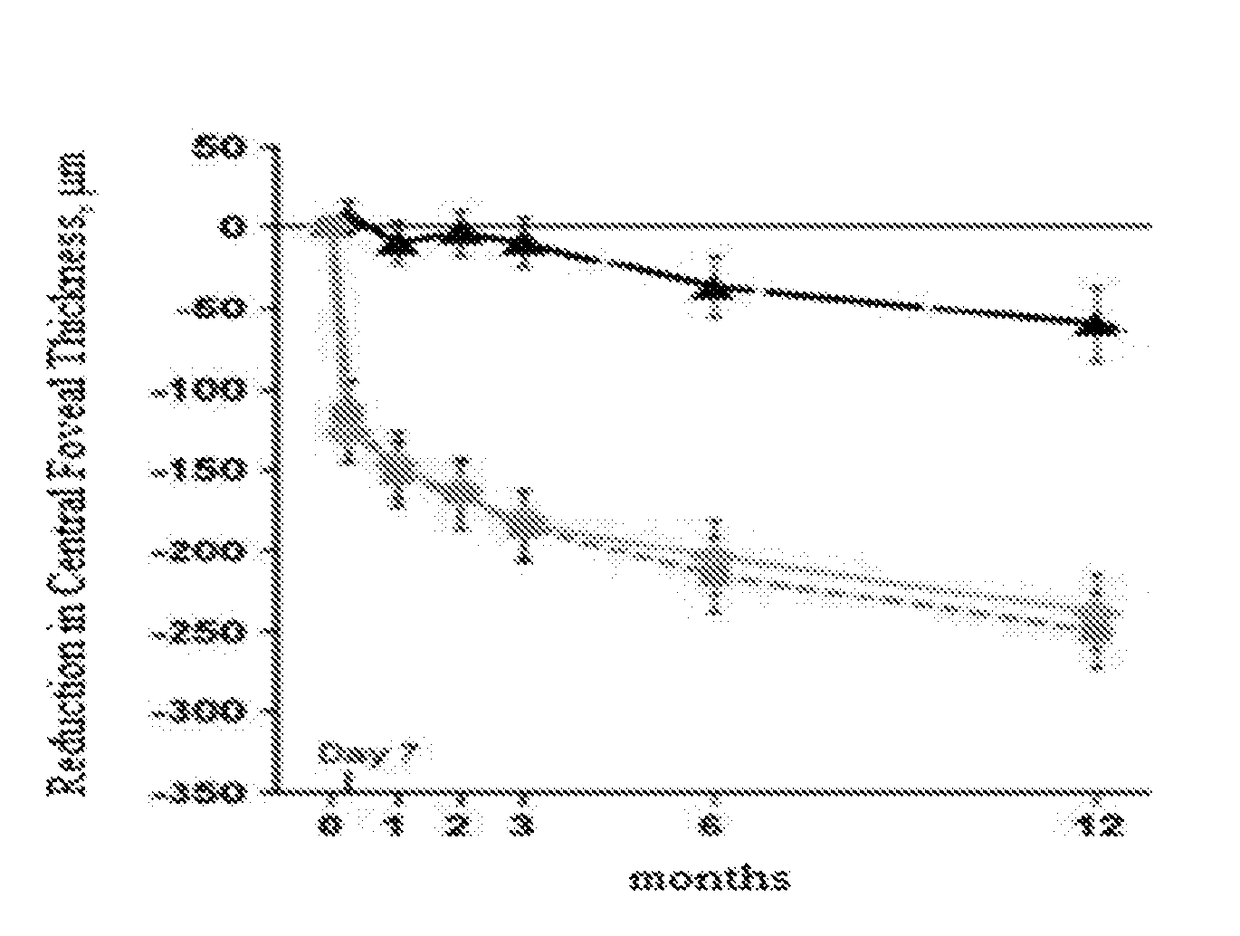
The present invention is a method of the present invention screens a patient with macular edema associated with visual loss for implantation of a sustained release drug-delivery implant that is less invasive than implanting a drug-delivery implant and is minimally invasive. The method comprises injecting a first steroid, eg. triamcinolone acetonide, having anti-inflammatory activity as a bolus into a patient's eye. Patients that experience a reduction in swelling in the retina (typically the macula and preferably the fovia) and improved vision are identified as candidate patients. Candidate patients are patients that are more likely to benefit from the implantation of a sustained release drug-delivery implant into the eye containing a second steroid, for example fluocinolone acetonide.
Owner:BAUSCH & LOMB INC
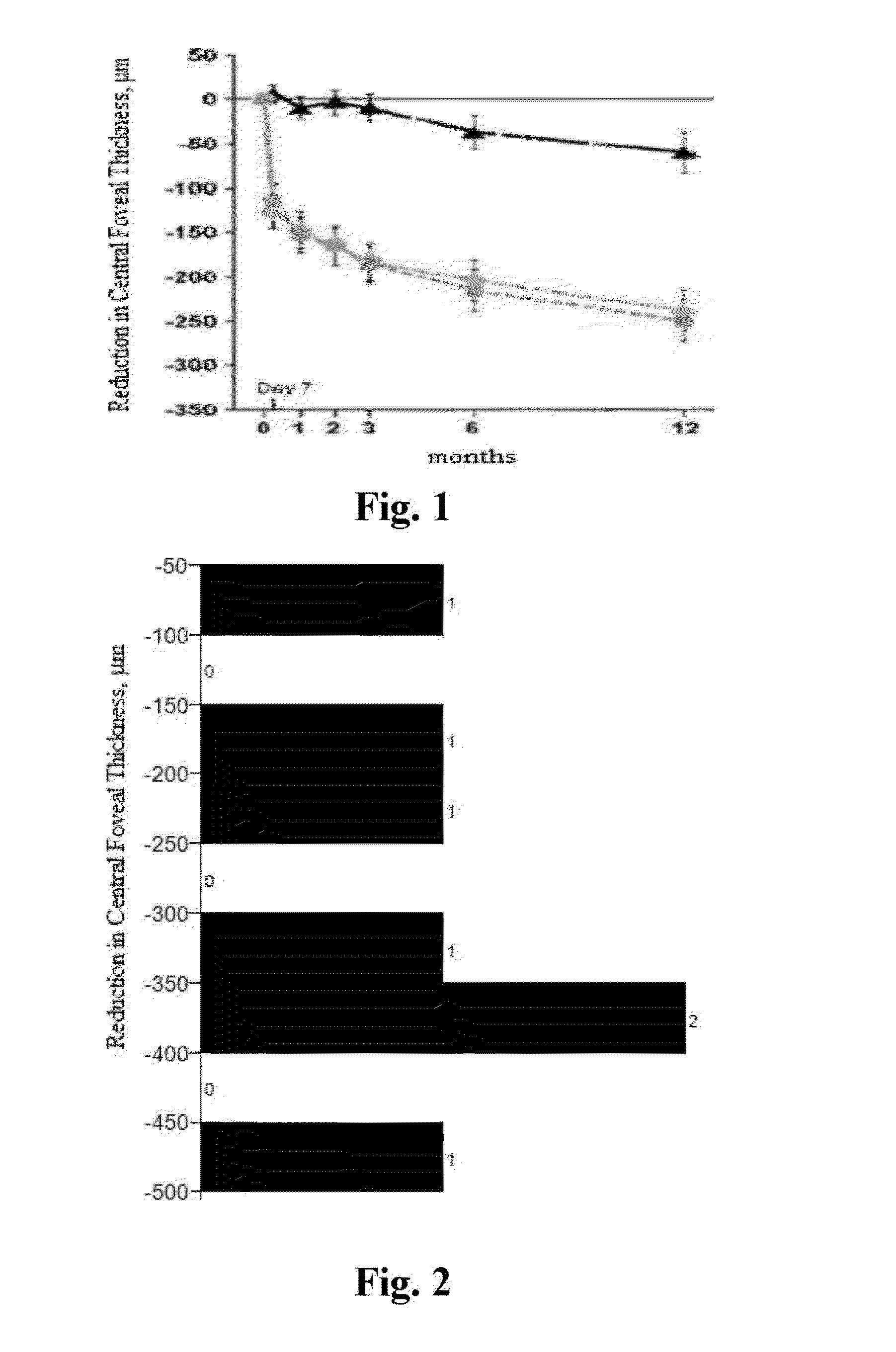
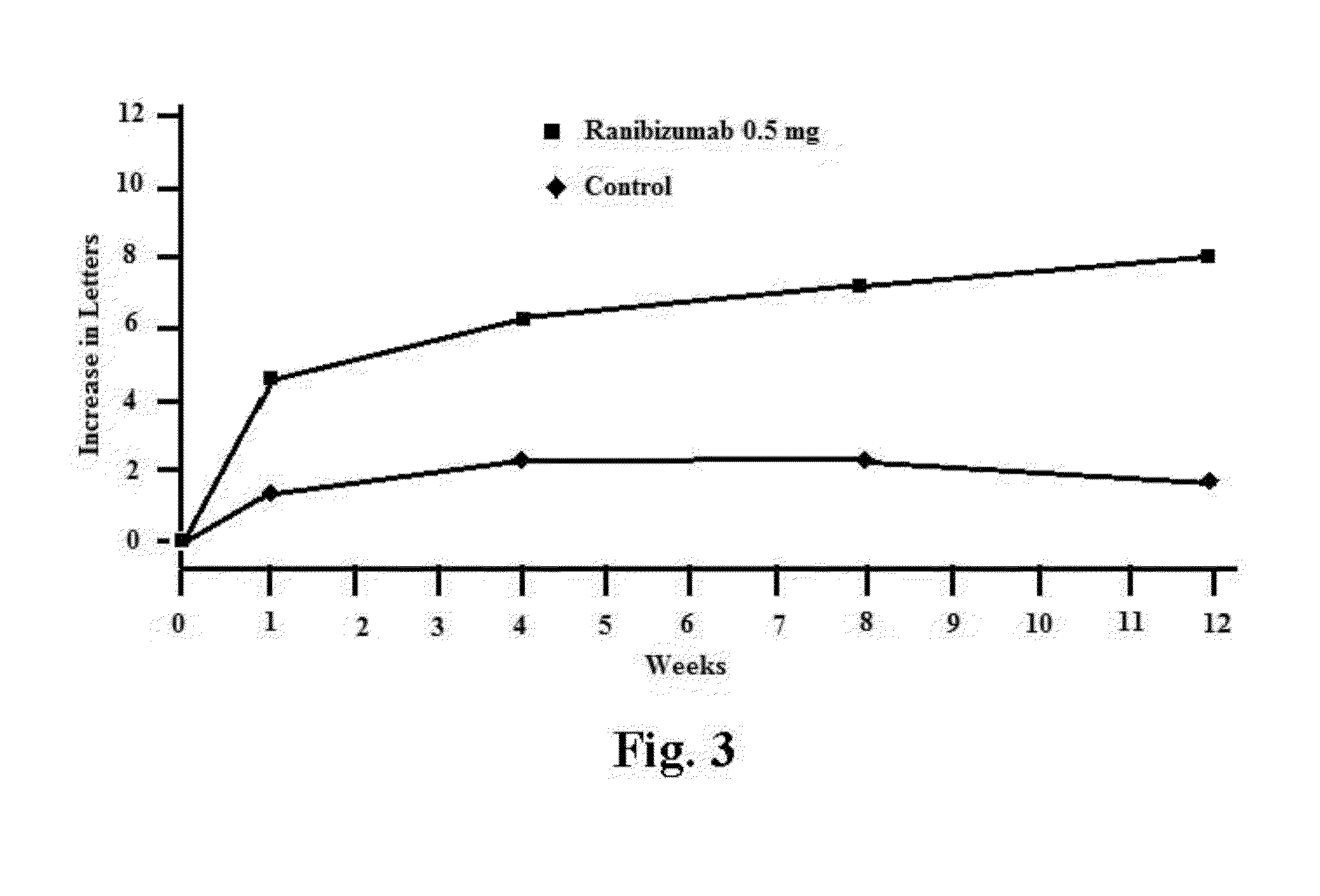
Methods for treating macular edema and ocular angiogenesis using an Anti-inflammatory agent and a receptor tyrosine kinase inhibitor
The present invention provides methods for inhibiting increased vascular permeability and / or pathologic ocular angiogenesis via administration of a combination of one or more molecules that potently inhibit select receptor tyrosine kinases (RTKs) or vascular endothelial growth factor (VEGF) and one or more anti-inflammatory agents.
Owner:NOVARTIS AG
Kinase inhibitors and methods of use thereof
InactiveUS7691858B2Inhibiting and reducing vascular leakageReduce leakageBiocideOrganic chemistryUveitisAutoimmune condition
Compositions and methods and are provided for treating disorders associated with compromised vasculostasis. Invention methods and compositions are useful for treating a variety of disorders including for example, stroke, myocardial infarction, cancer, ischemia / reperfusion injury, autoimmune diseases such as rheumatoid arthritis, eye diseases such as uveitis, retinopathies or macular degeneration, macular edema or other vitreoretinal diseases, inflammatory diseases such as autoimmune diseases, vascular leakage syndrome, edema, or diseases involving leukocyte activation, transplant rejection, respiratory diseases such as asthma, adult or acute respiratory distress syndrome (ARDS), chronic obstructive pulmonary disease, and the like.
Owner:TARGEGEN
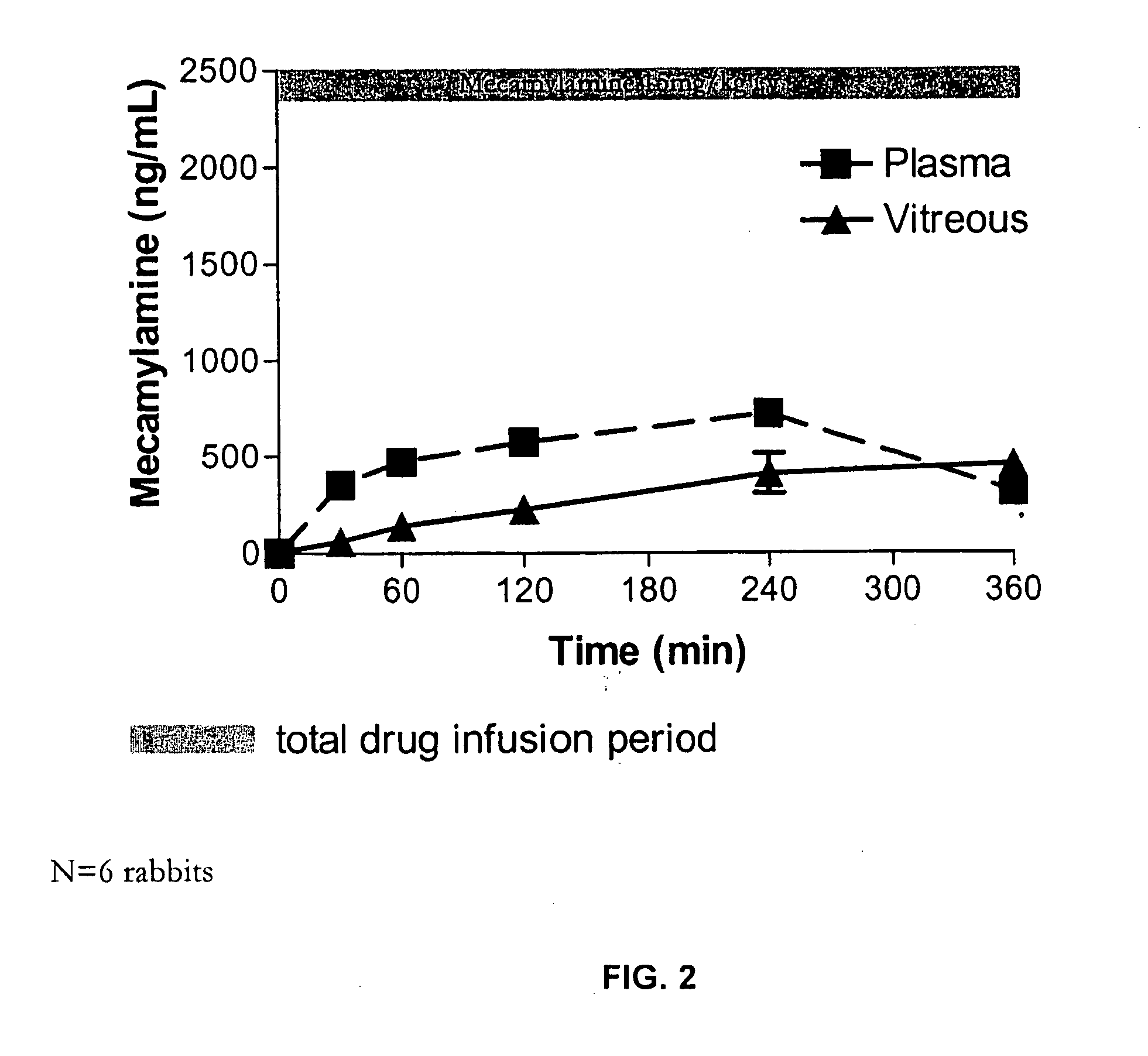
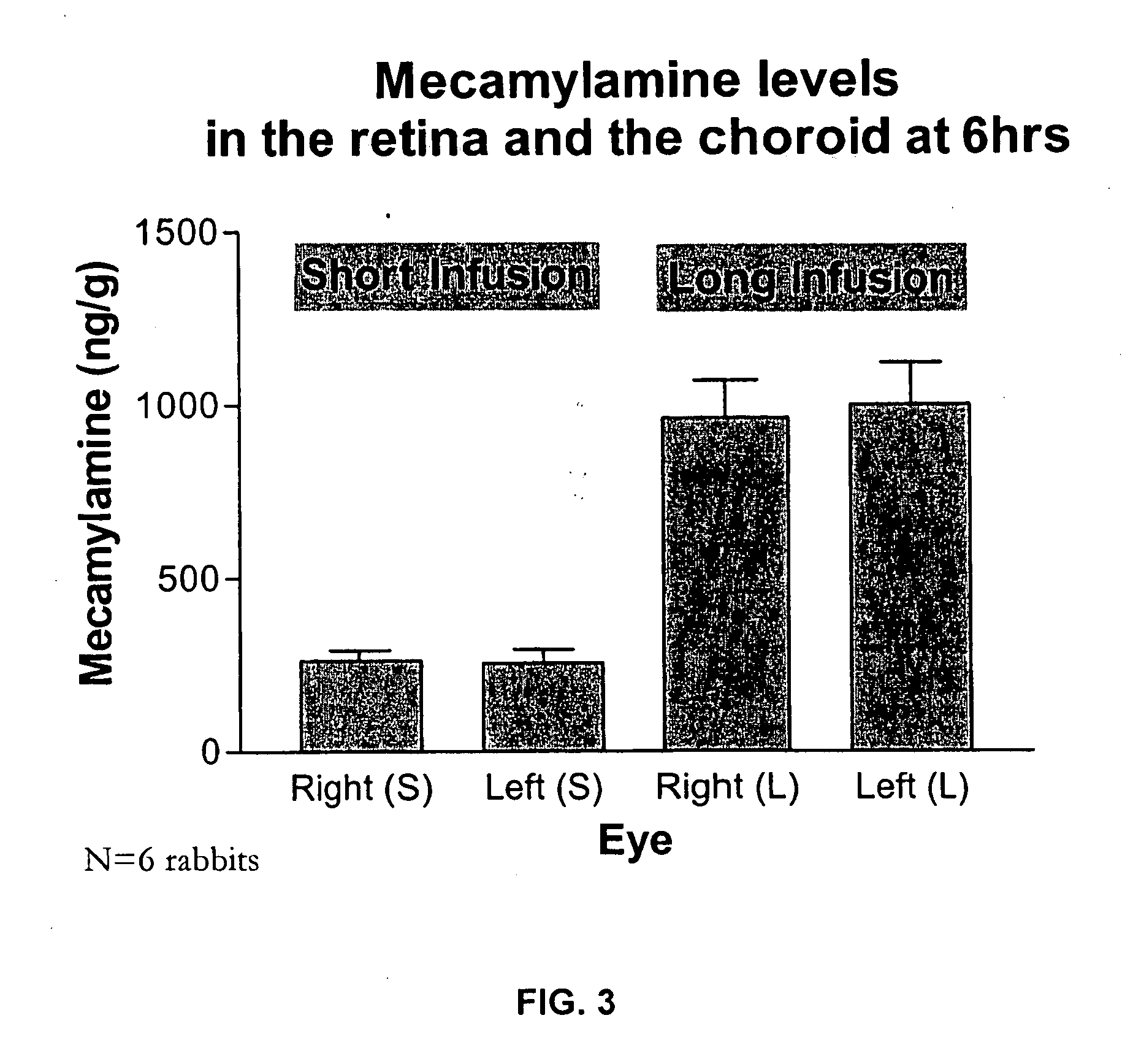
Topical mecamylamine formulations for ocular administration and uses thereof
Provided are methods, pharmaceutical formulations and kits thereof for the treatment and / or prevention of conditions mediated by neovascularization, abnormal angiogenesis, vascular permeability, or combinations thereof, of posterior and / or anterior tissues and fluids of the eye, including conditions associated with proliferative retinopathies, for example, diabetic retinopathy, age-related maculopathy, retinopathy of prematurity, retinopathy associated with macular edema, or retinopathy associated with sickle cell disease, using the topical administration of mecamylamine or a pharmaceutically acceptable salt thereof to the eye. Methods of preparing the pharmaceutical formulations are also provided.
Owner:COMENTIS
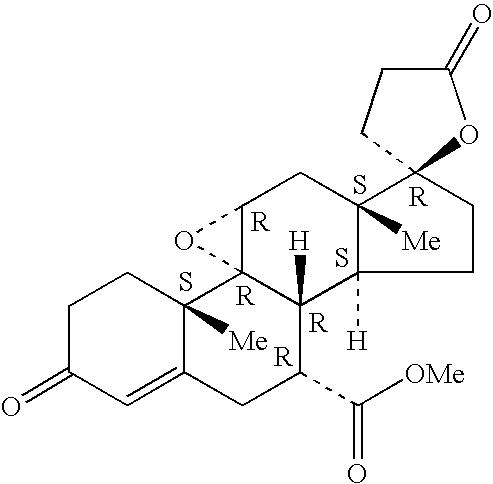
Methods of treating ophthalmic disorders with epoxy-steroidal aldosterone receptor antagonists
Owner:PHARMACIA CORP
Novel Angiopoietin 2, VEGF Dual Antagonists
ActiveUS20170327569A1Inhibit bindingAvoid blindnessAntibody mimetics/scaffoldsImmunoglobulins against growth factorsNeovascular glaucomaVEGF receptors
The present disclosure relates to chimeric molecules which are fusion proteins comprising two components: an Ang-2 binding peptide linked to either a VEGF antibody or a VEGF receptor-Fc fusion protein. The Ang2 peptide, VEGF antibody, and VEGF receptor-Fc fusion proteins are each defined below with reference to percent identity to a reference sequence. The chimeric molecule is a fusion protein, dual antagonist of Ang2 and VEGF for treatment of cancers, proliferative retinopathies, neovascular glaucoma, macular edema, AMD, and rheumatoid arthritis.
Owner:ASKGENE PHARM INC
Methods and Compositions for Treatment of Macular and Retinal Disease
InactiveUS20070259843A1Convenient treatmentImprove efficacyBiocideSenses disorderExudative age-related macular degenerationDisease cause
The present invention describes linking a therapeutic agent to a compound which is known to be naturally concentrated in a tissue affected by, or that is causing, a disease, to create a prodrug for treatment of the disease. Embodiments of the present invention include a new class of carotenoid-linked drugs to treat such blinding retinal disease such as age-related macular degeneration, retinoblastoma, and diabetic macular edema. For example, the present invention comprises a method for the treatment of a disorder of the eye comprising linking a therapeutic agent to a xanthophyll carotenoid to create a prodrug, and administering a therapeutically effective amount of the prodrug to an individual in need of treatment. Provided are prodrugs for treatment of retinoblastoma, cystoid macular edema (CME), exudative age-related macular degeneration (AMD), diabetic retinopathy, diabetic macular edema, or inflammatory disorders.
Owner:UNIV OF GEORGIA RES FOUND INC
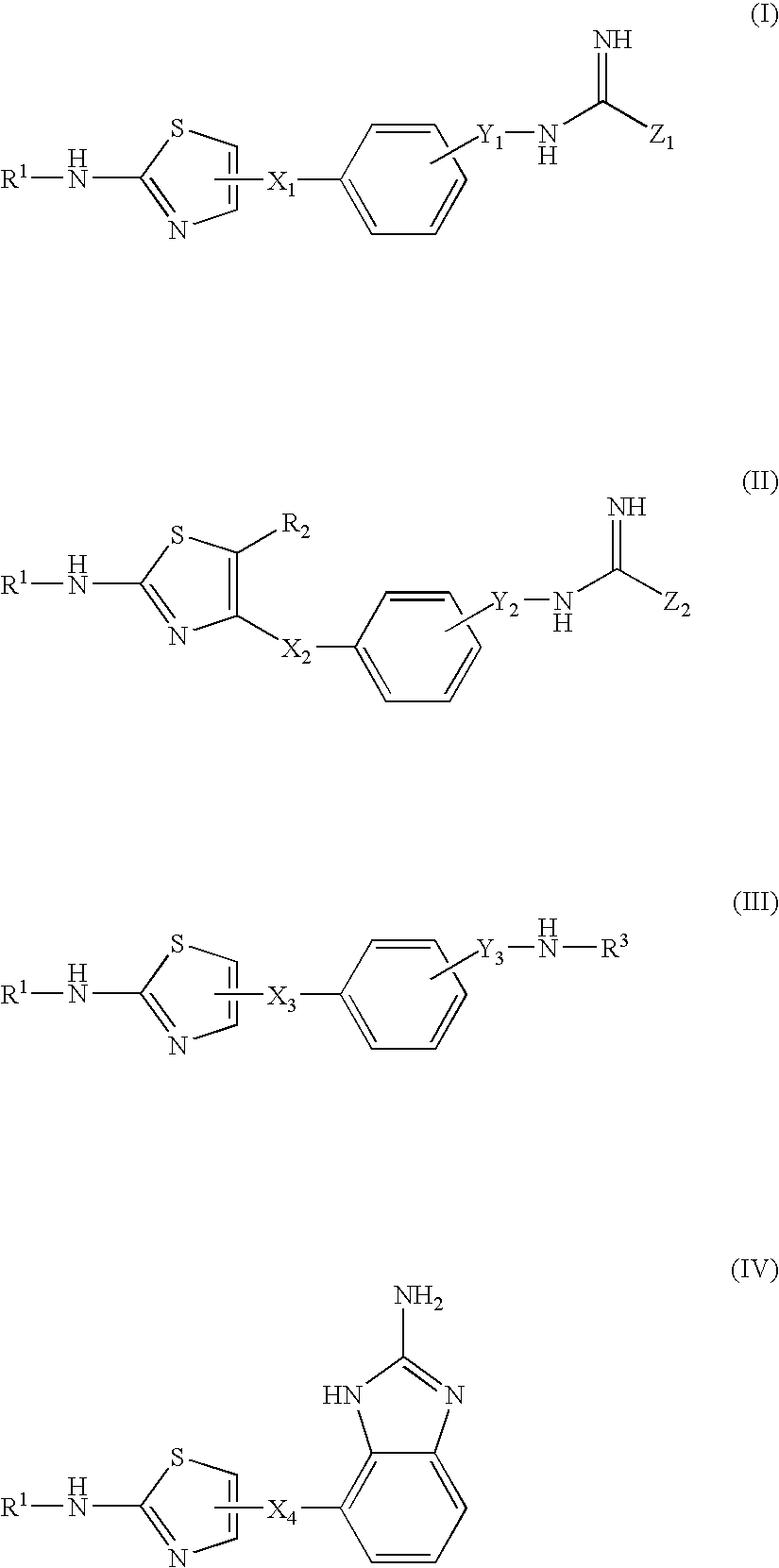
Thiazole Derivatives Having Vap-1 Inhibitory Activity
A compound of the formula (I), (II), (III) or (IV): wherein each symbol is as defined in the specification, or a pharmaceutically acceptable salt thereof useful as a vascular adhesion protein-1 (VAP-1) inhibitor, a pharmaceutical composition, a method for preventing or treating a VAP-1 associated disease, especially macular edema, which method includes administering an effective amount of the compound or a pharmaceutically acceptable salt thereof to a subject, and the like.
Owner:ASTELLAS PHARMA INC +1
Compositions and methods for treating noninfectious uveitis
InactiveUS20190269702A1Reduce inflammationReduces inflammatory scoreOrganic active ingredientsSenses disorderUveitisMacular edema
The present invention relates to methods, devices, and compositions for treating ocular disorders such as uveitis, macular edema associated with uveitis, and diabetic macular edema. For example, the methods include treatment of subjects having macular edema associated with non-infectious uveitis, or diabetic macular edema, by administering to the subjects a triamcinolone composition via non-surgical administration to the suprachoroidal space (SCS) of the eye.
Owner:CLEARSIDE BIOMEDICAL
Therapeutic agent for ocular disease or prophylactic agent for ocular disease
ActiveCN105636590AImprove permeabilityOrganic active ingredientsSenses disorderDiabetic retinopathyRetinal blood vessels
3-[(3S,4R)-3-methyl-6-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1,6-diazaspiro[3.4]octan-1-yl]-3-oxopropanenitrile suppresses increases in retinal blood vessel permeability induced by VEGF and therefore can be used as an active component in therapeutic agents or prophylactic agents for a variety of ocular diseases to which VEGF contributes, such as age-related macular degeneration, diabetic retinopathy, macular edema, neovascular maculopathy, retinal vein occlusion, and neovascular glaucoma.
Owner:JAPAN TOBACCO INC
Compositions and methods for treating ocular diseases
ActiveUS20170319602A1Dipeptide ingredientsNitro compound active ingredientsUveitisMacula lutea degeneration
Owner:EYEPOINT PHARMA INC
Compositions and Methods for Treating Ophthalmic Diseases
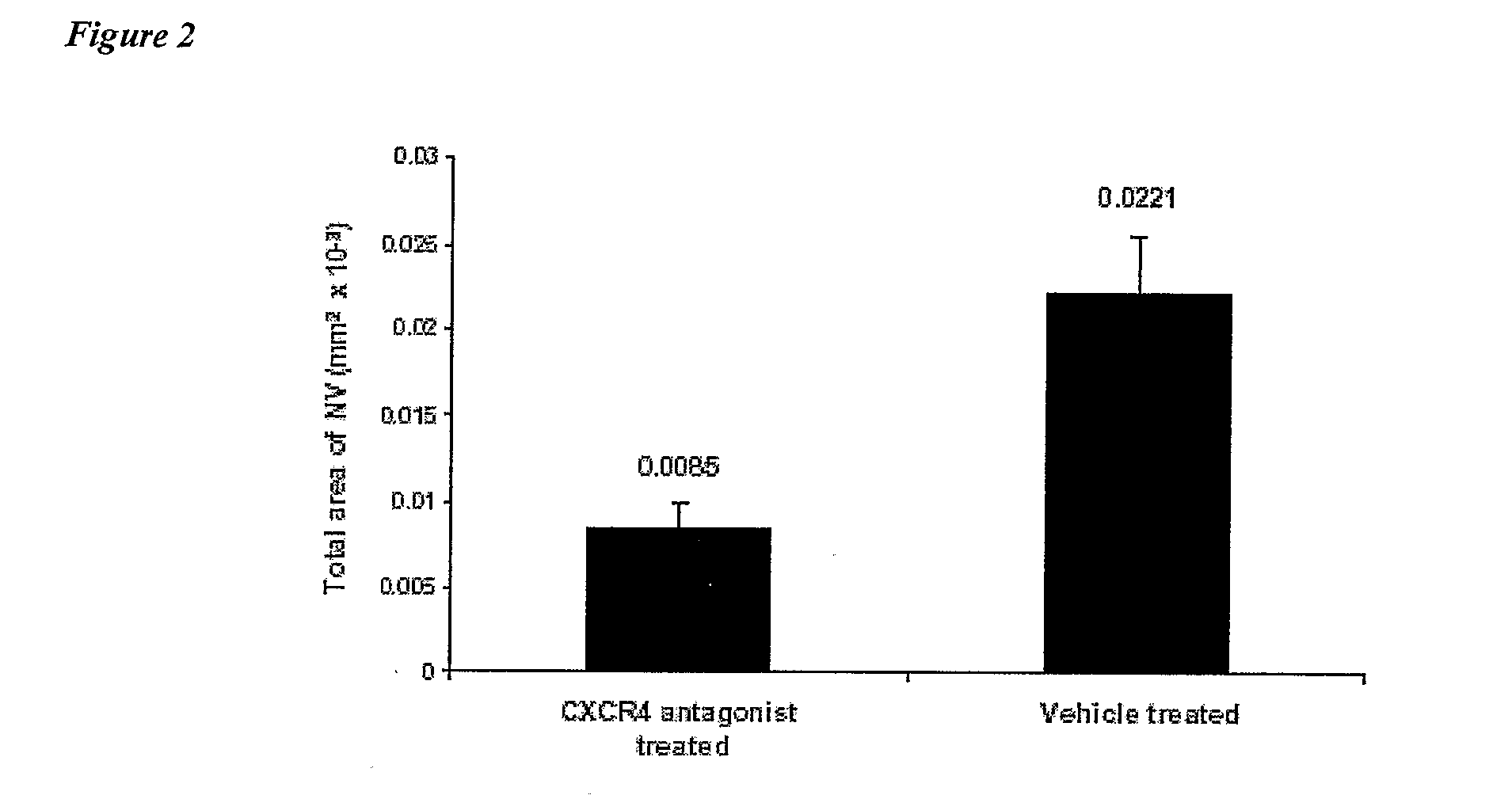
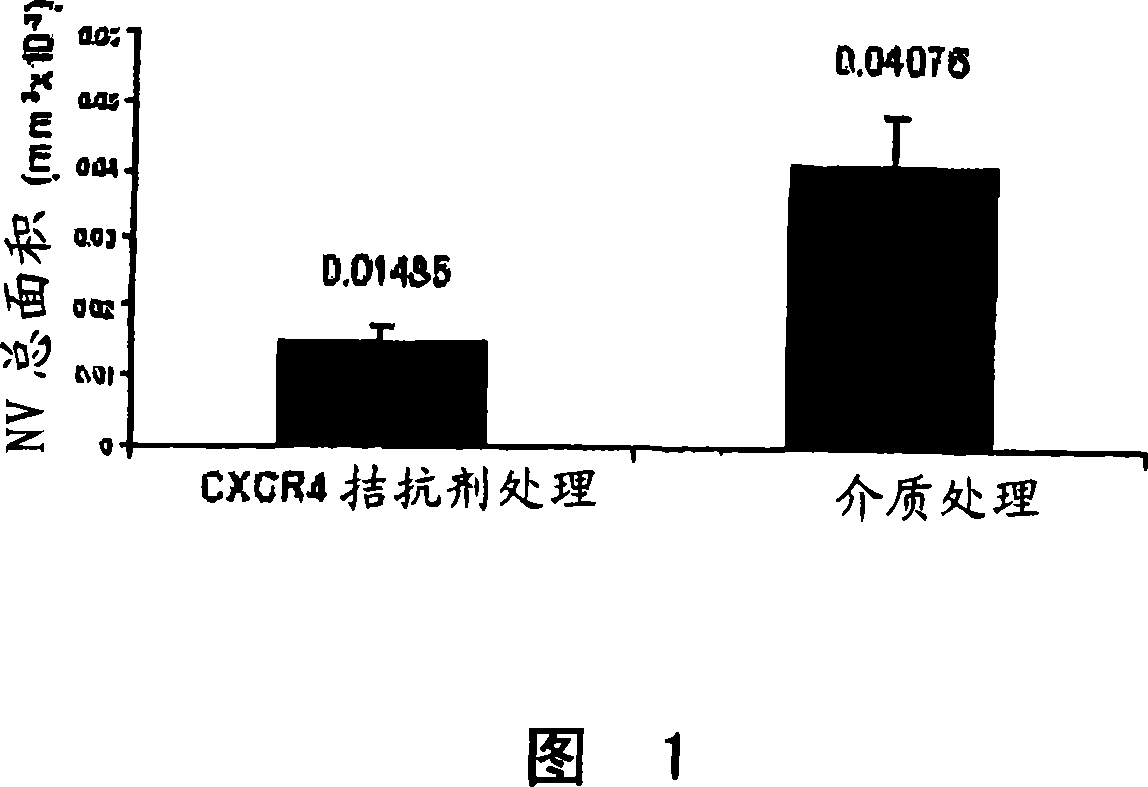
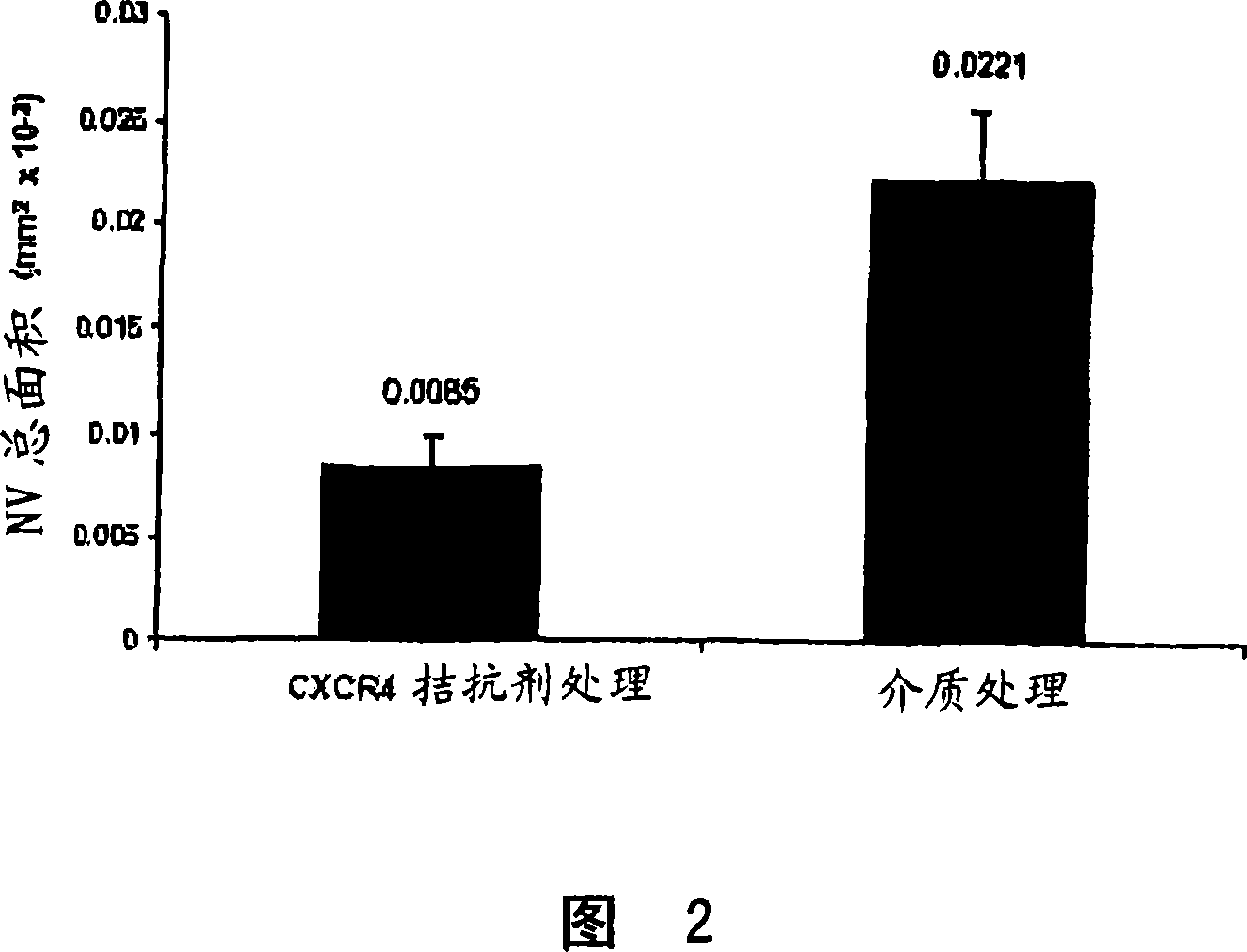
This invention relates to CXCR4 inhibitors and their use in treating and / or preventing a variety of angiogenic, microvascular and ocular disorders including primary indications for diabetic retinopathy, macular degeneration (such as wet or neovascular age-related macular degeneration (AMD) and dry or atrophic AMD), macular edema, and secondary indications for inhibiting tumor vascularization, and corneal and iris neovascularization.
Owner:MERCK SHARP & DOHME CORP
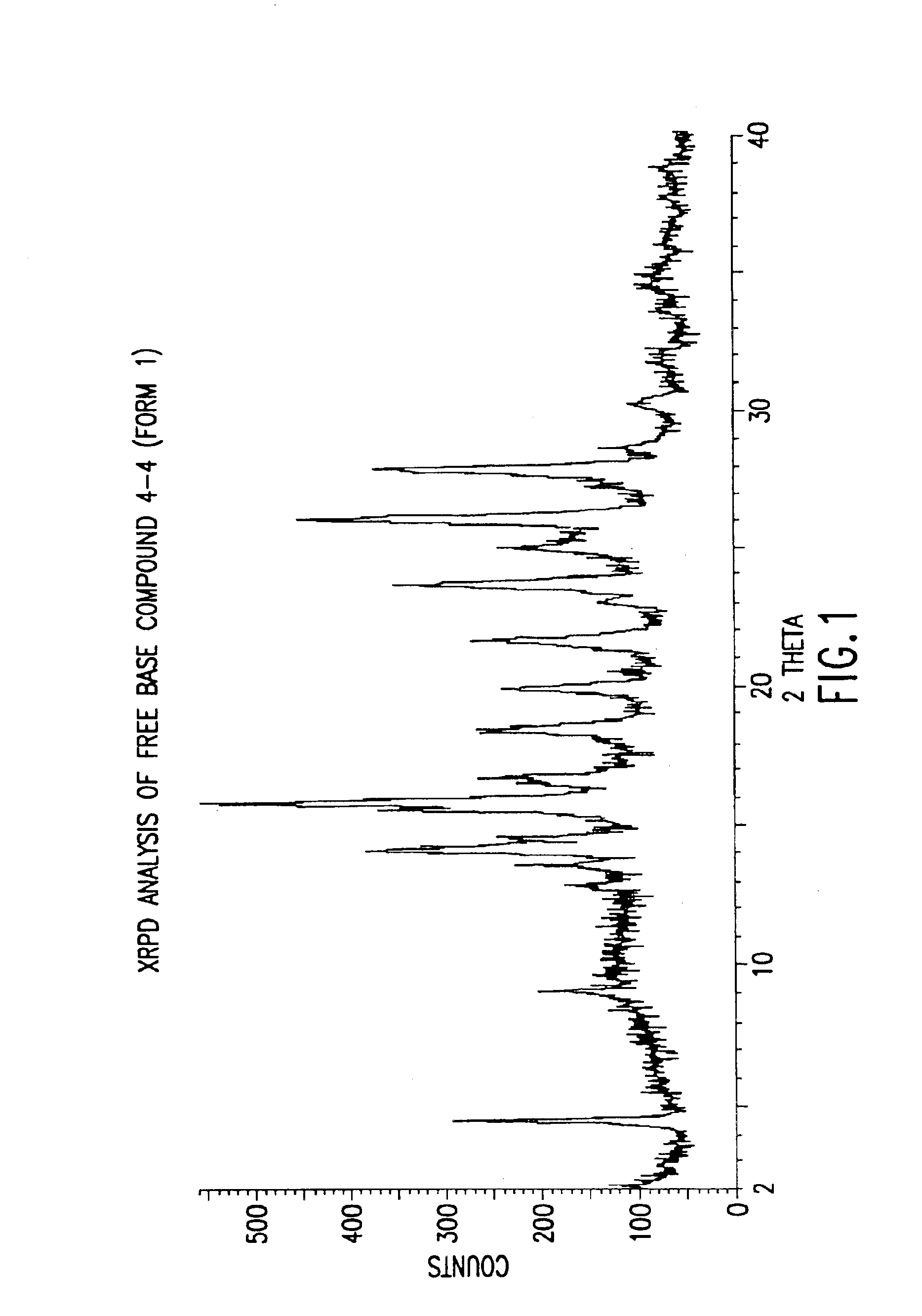
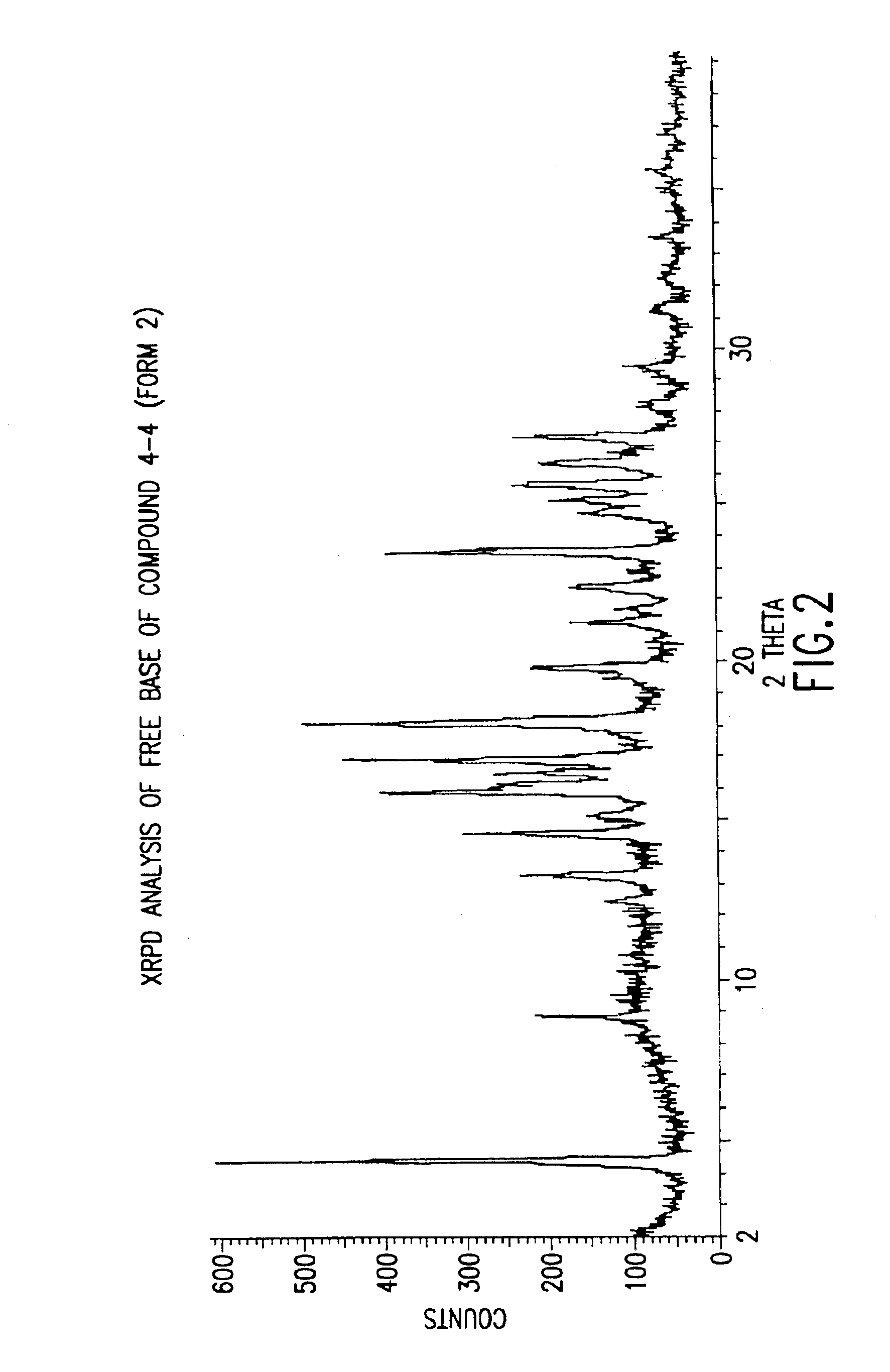
Polymorphs with tyrosine kinase activity
The present invention relates to active polymorphs of 4-[2-(5-cyano-thiazol-2-ylamino)-pyridin-4-ylmethyl]-piperazine-1-carboxylic acid methylamide which inhibit, regulate and / or modulate tyrosine kinase signal transduction, compositions which contain these compounds, and methods of using them to treat tyrosine kinase-dependent diseases and conditions, such as angio-genesis, cancer, tumor growth, atherosclerosis, age related macular degeneration, diabetic retinopathy, retinal ischemia, macular edema, inflammatory diseases, and the like in mammals.
Owner:MERCK SHARP & DOHME CORP
Compositions and methods for treating ophthalmic diseases
InactiveCN101035537ASuppress generationOrganic active ingredientsSenses disorderDiseaseIris neovascularization
This invention relates to CXCR4 inhibitors and their use in treating and / or preventing a variety of angiogenic, microvascular and ocular disorders including primary indications for diabetic retinopathy, macular degeneration (such as wet or neovascular age-related macular degeneration (AMD) and dry or atrophic AMD), macular edema, and secondary indications for inhibiting tumor vascularization, and corneal and iris neovascularization.
Owner:MERCK & CO INC
Tyrosine kinase inhibitors
The present invention relates to compounds which inhibit, regulate and / or modulate tyrosine kinase signal transduction, compositions which contain these compounds, and methods of using them to treat tyrosine kinase-dependent diseases and conditions, such as angiogenesis, cancer, tumor growth, atherosclerosis, age related macular degeneration, diabetic retinopathy, macular edema, retinal ischemia, inflammatory diseases, and the like in mammals.
Owner:MERCK SHARP & DOHME CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Substituted pyrazolo[1,5-A]pyrimidines as tyrosine kinase inhibitors Substituted pyrazolo[1,5-A]pyrimidines as tyrosine kinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3d33d8fe-d3f5-4ead-a5b0-e3bb9a3e22d6/US07550470-20090623-C00001.png)
![Substituted pyrazolo[1,5-A]pyrimidines as tyrosine kinase inhibitors Substituted pyrazolo[1,5-A]pyrimidines as tyrosine kinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3d33d8fe-d3f5-4ead-a5b0-e3bb9a3e22d6/US07550470-20090623-C00002.png)
![Substituted pyrazolo[1,5-A]pyrimidines as tyrosine kinase inhibitors Substituted pyrazolo[1,5-A]pyrimidines as tyrosine kinase inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/3d33d8fe-d3f5-4ead-a5b0-e3bb9a3e22d6/US07550470-20090623-C00003.png)