Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
324 results about "Calibration set" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Calibration is setting devices to an appropriate set of values. Profiling is a record of the colours available in a device whether that is your camera, printer, or monitor. Using profiles aids the translation of colour from one device to another.
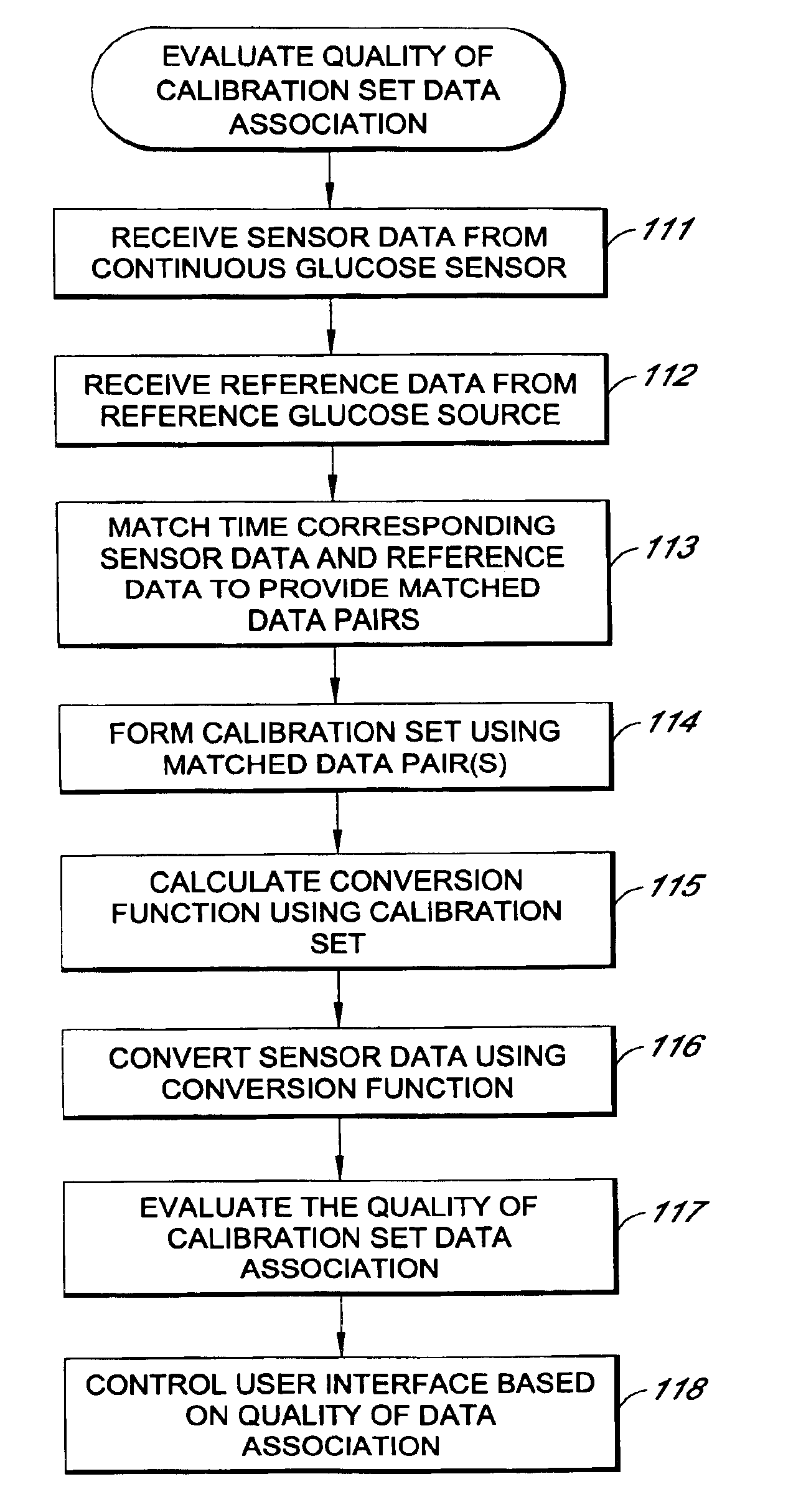
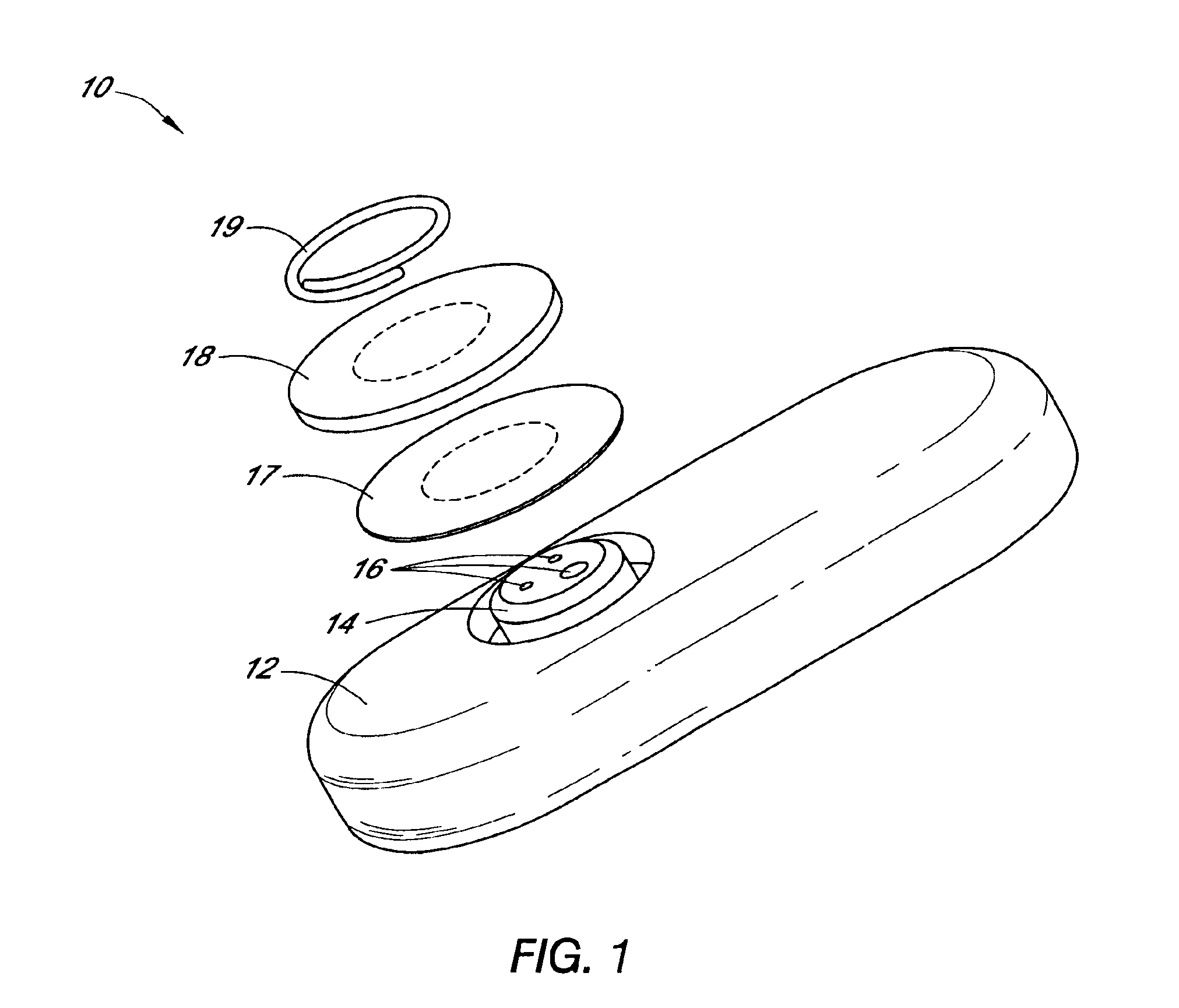
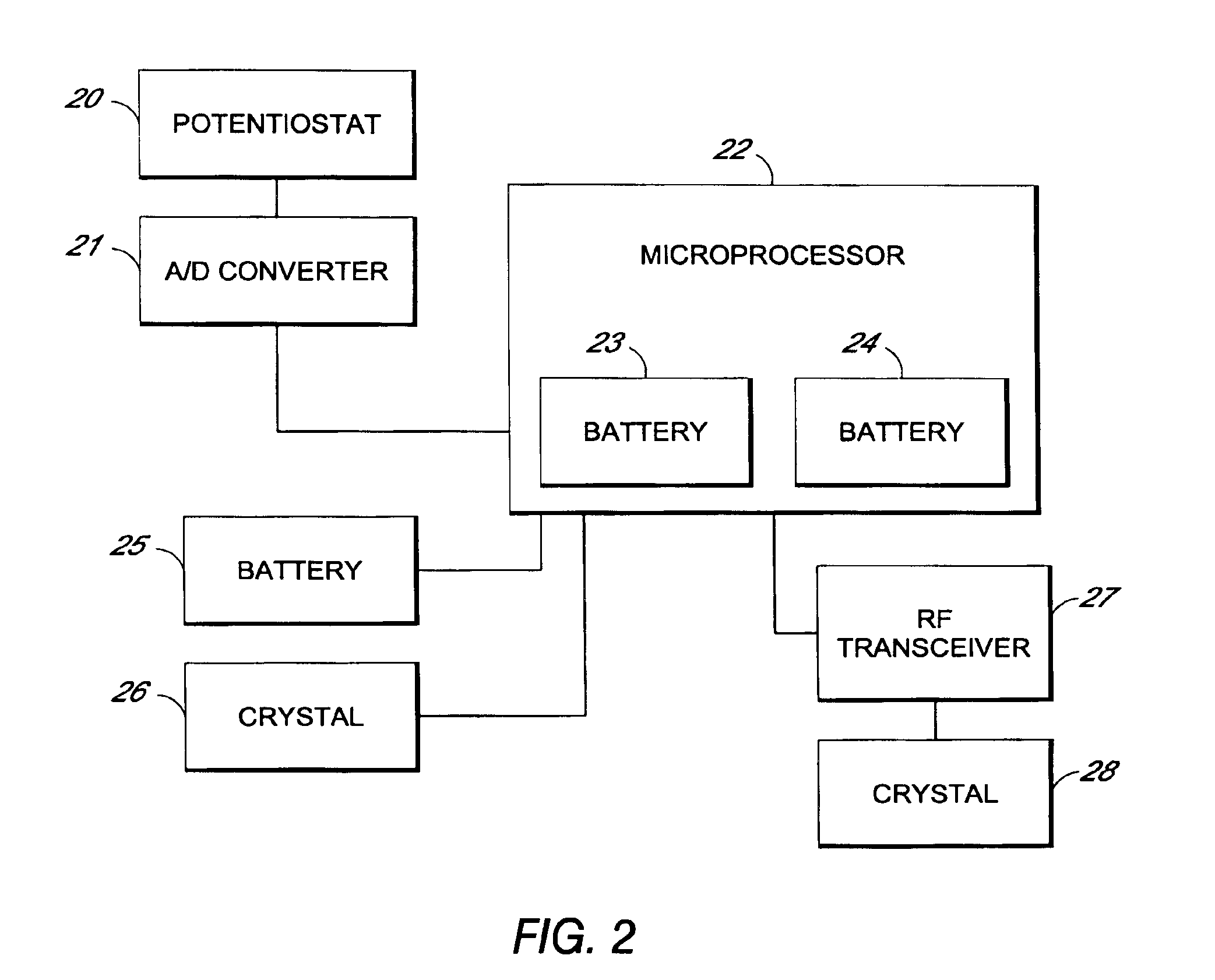
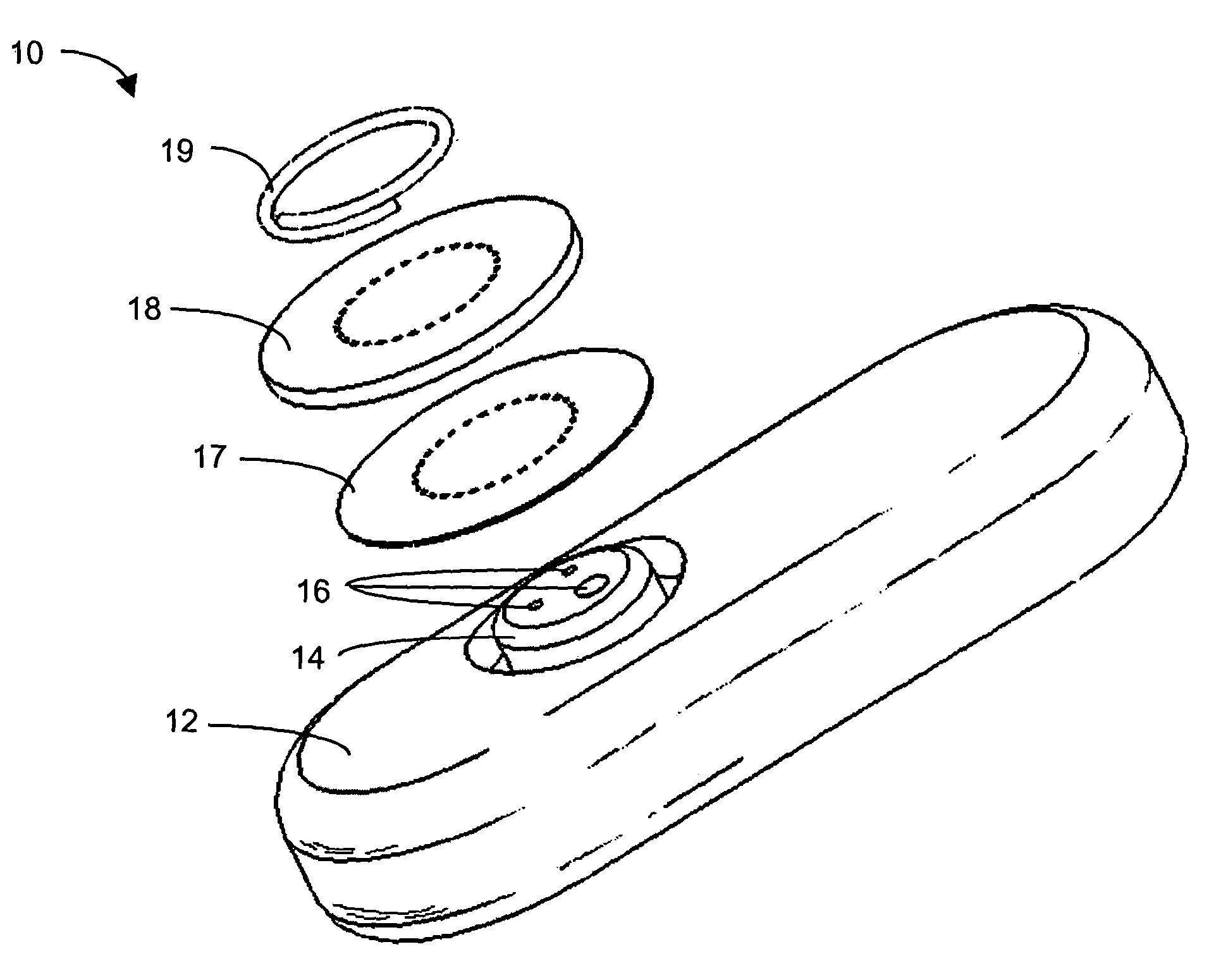
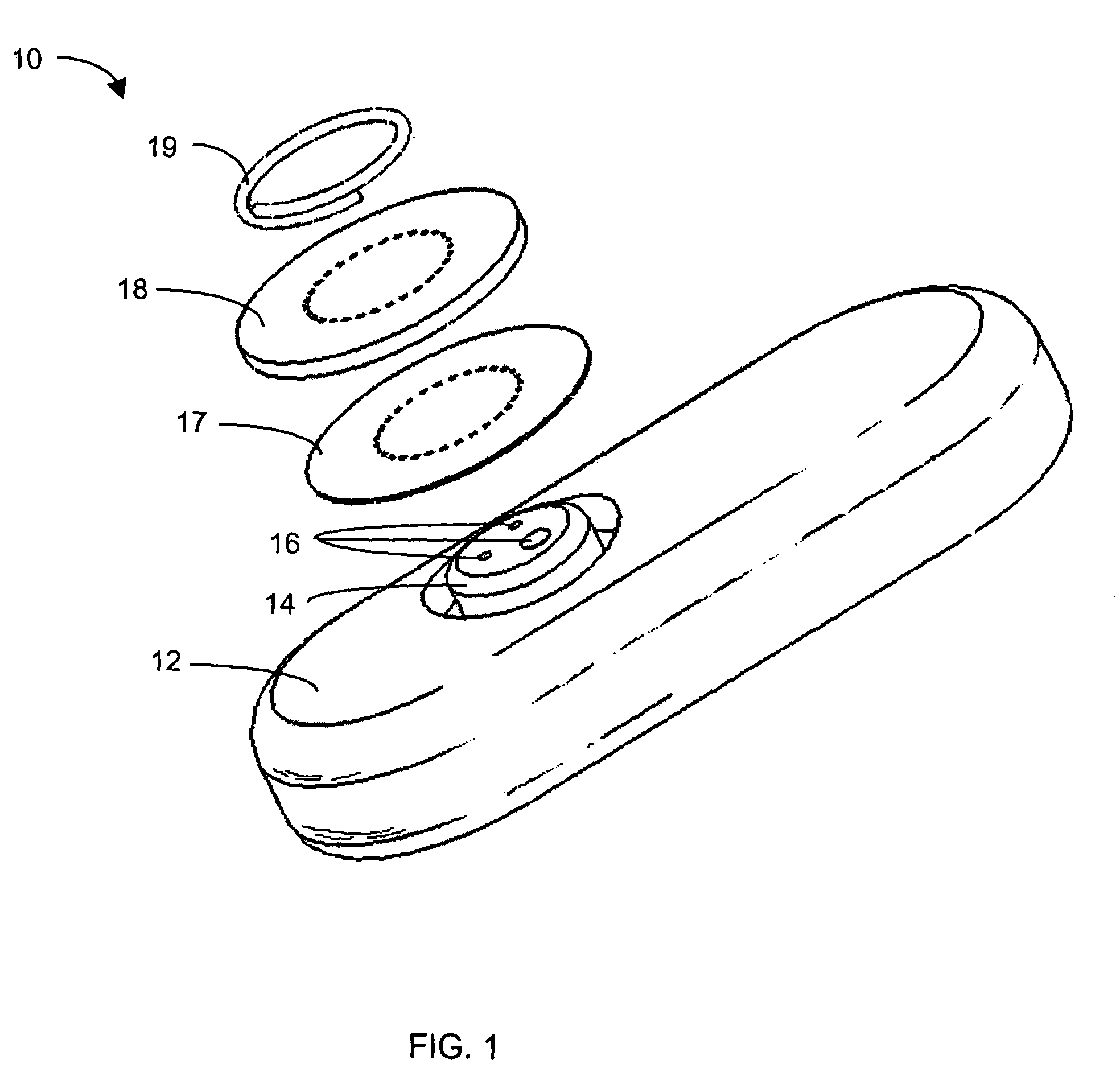
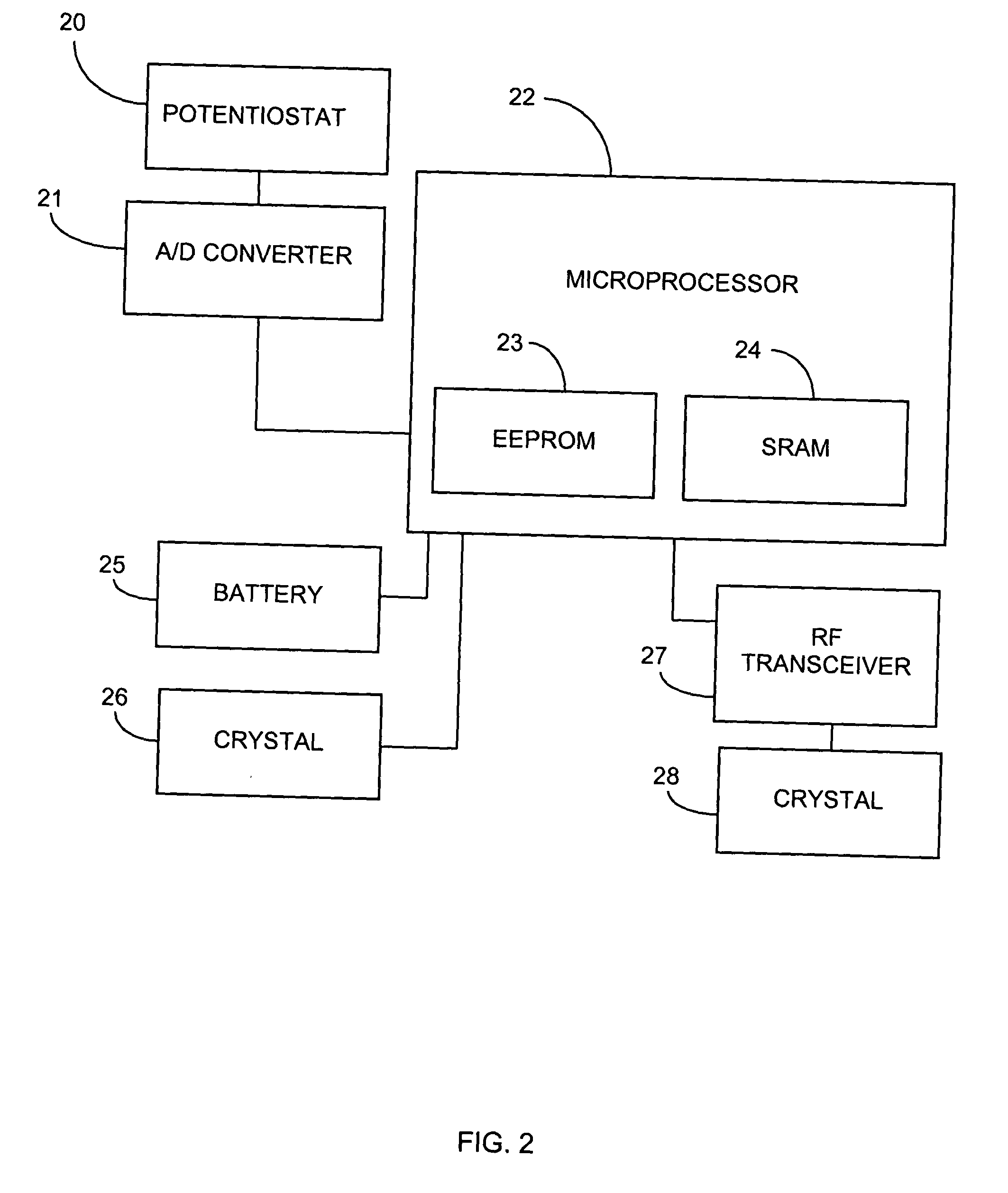
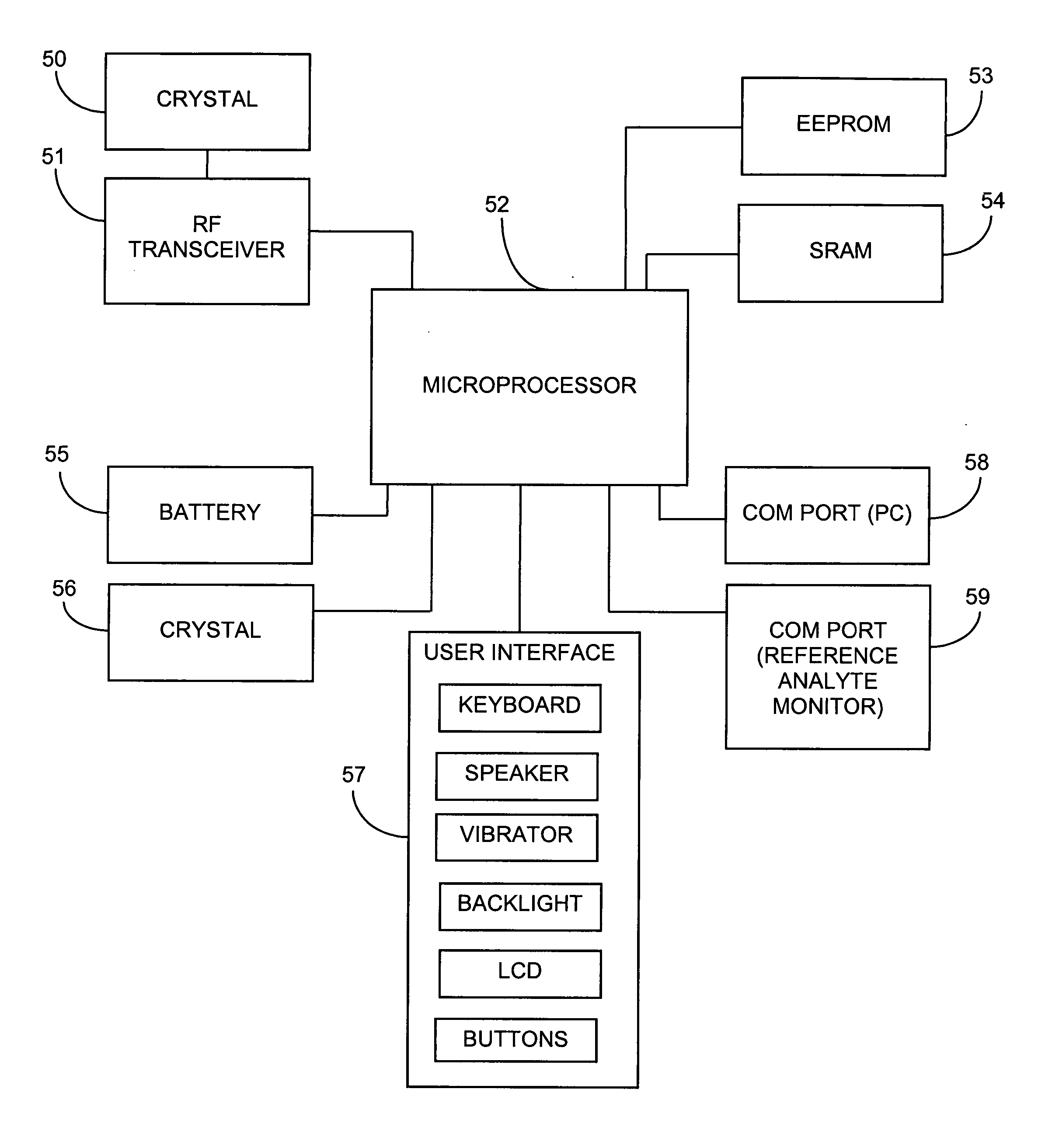
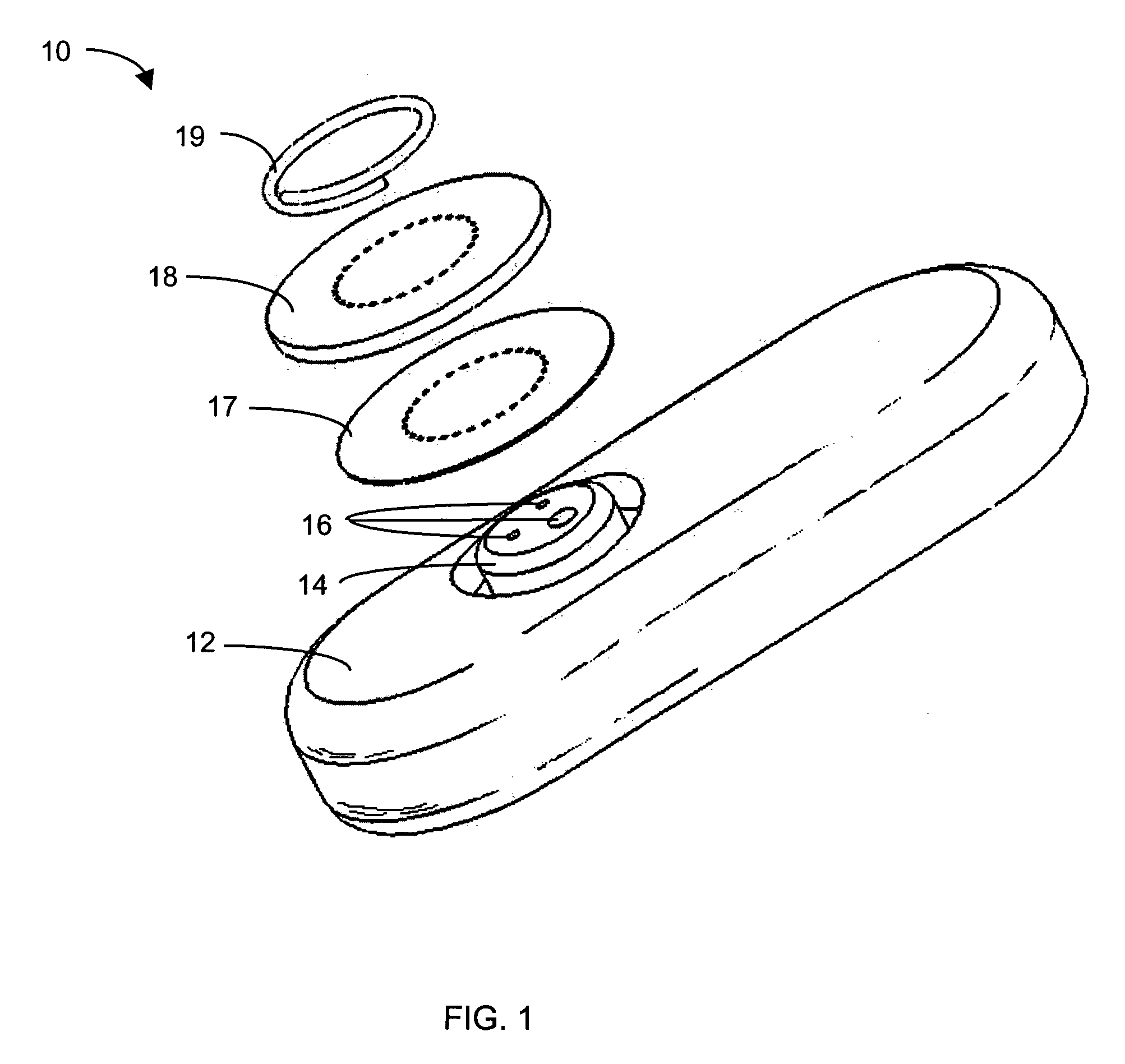
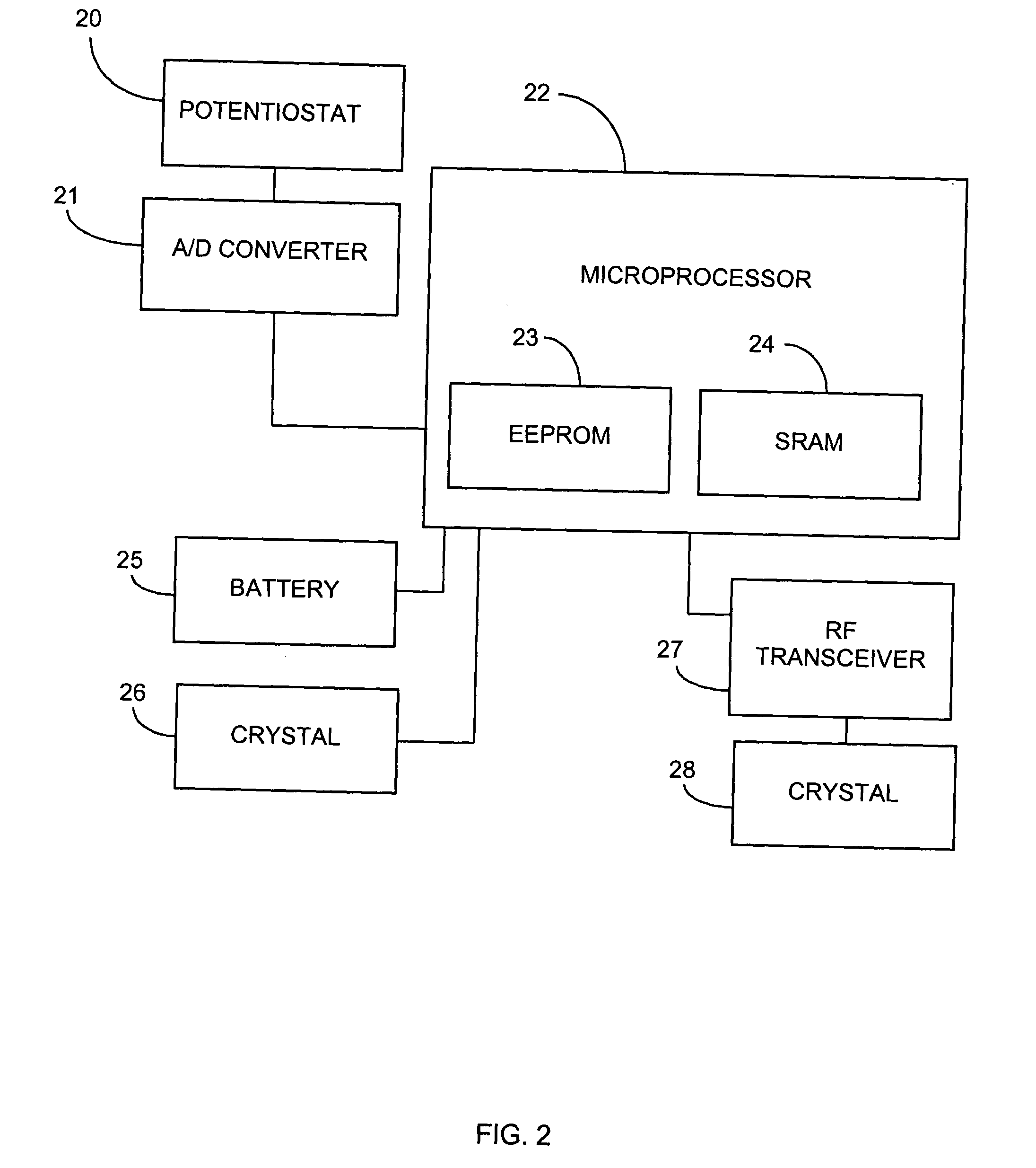
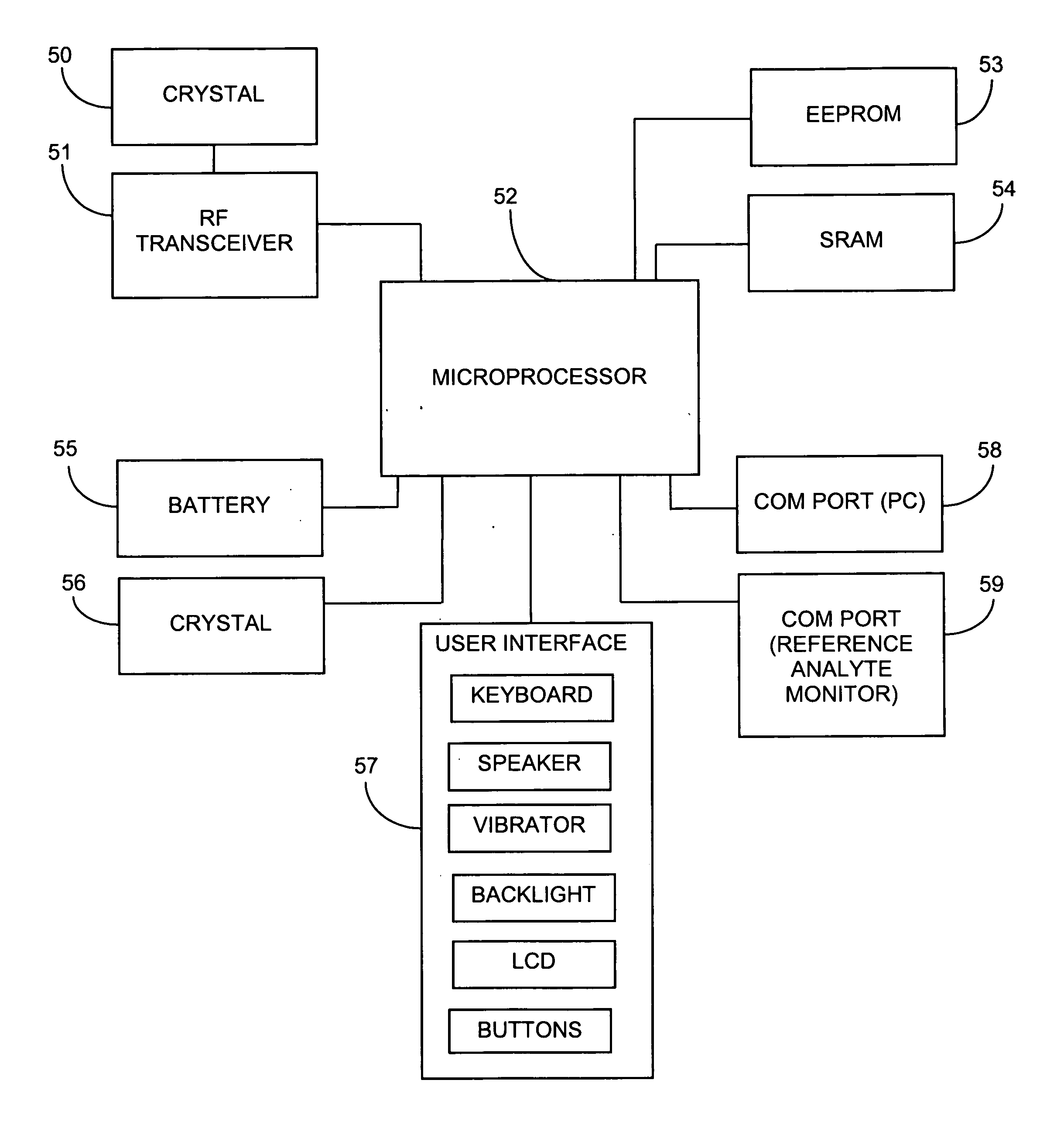
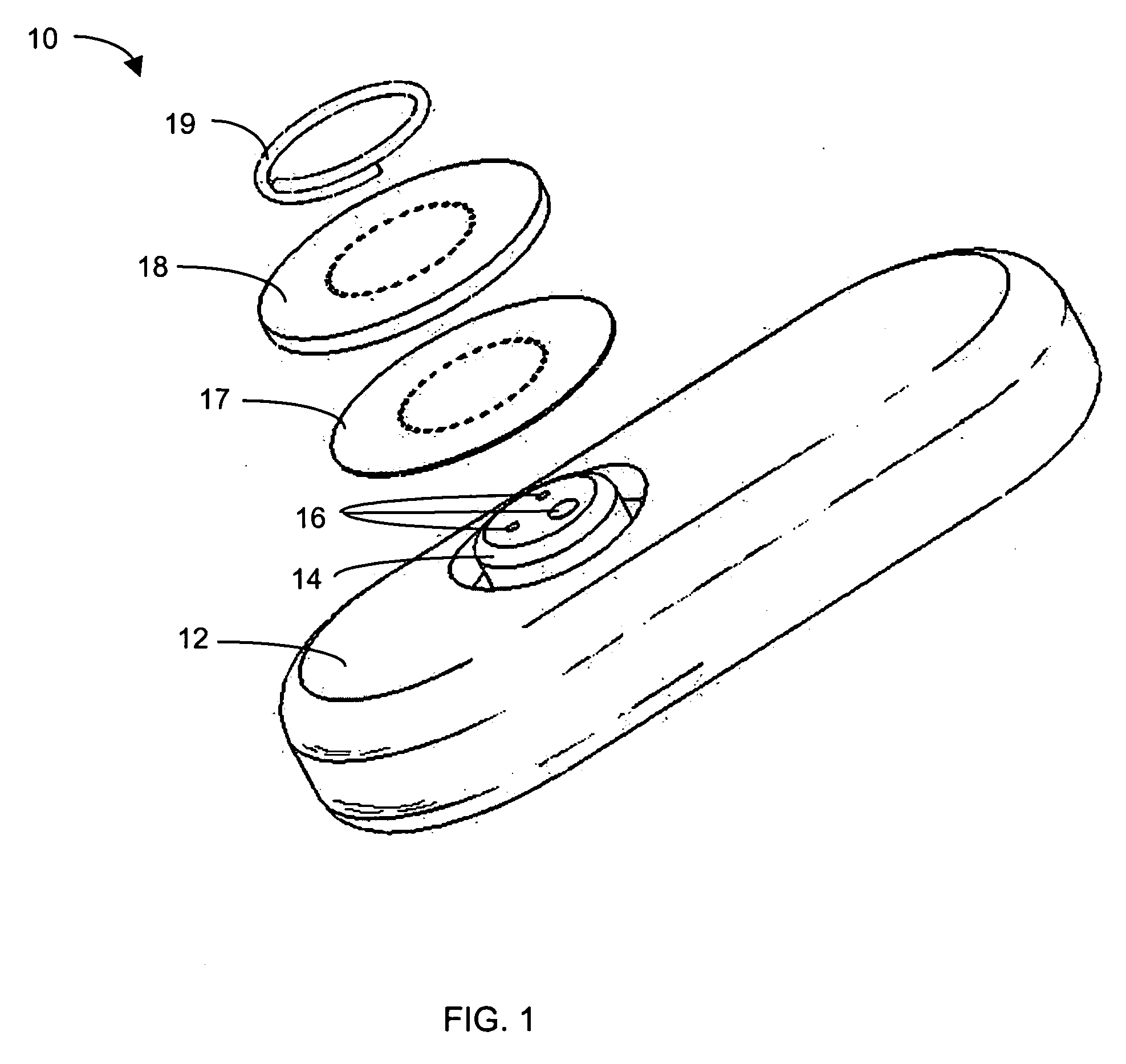
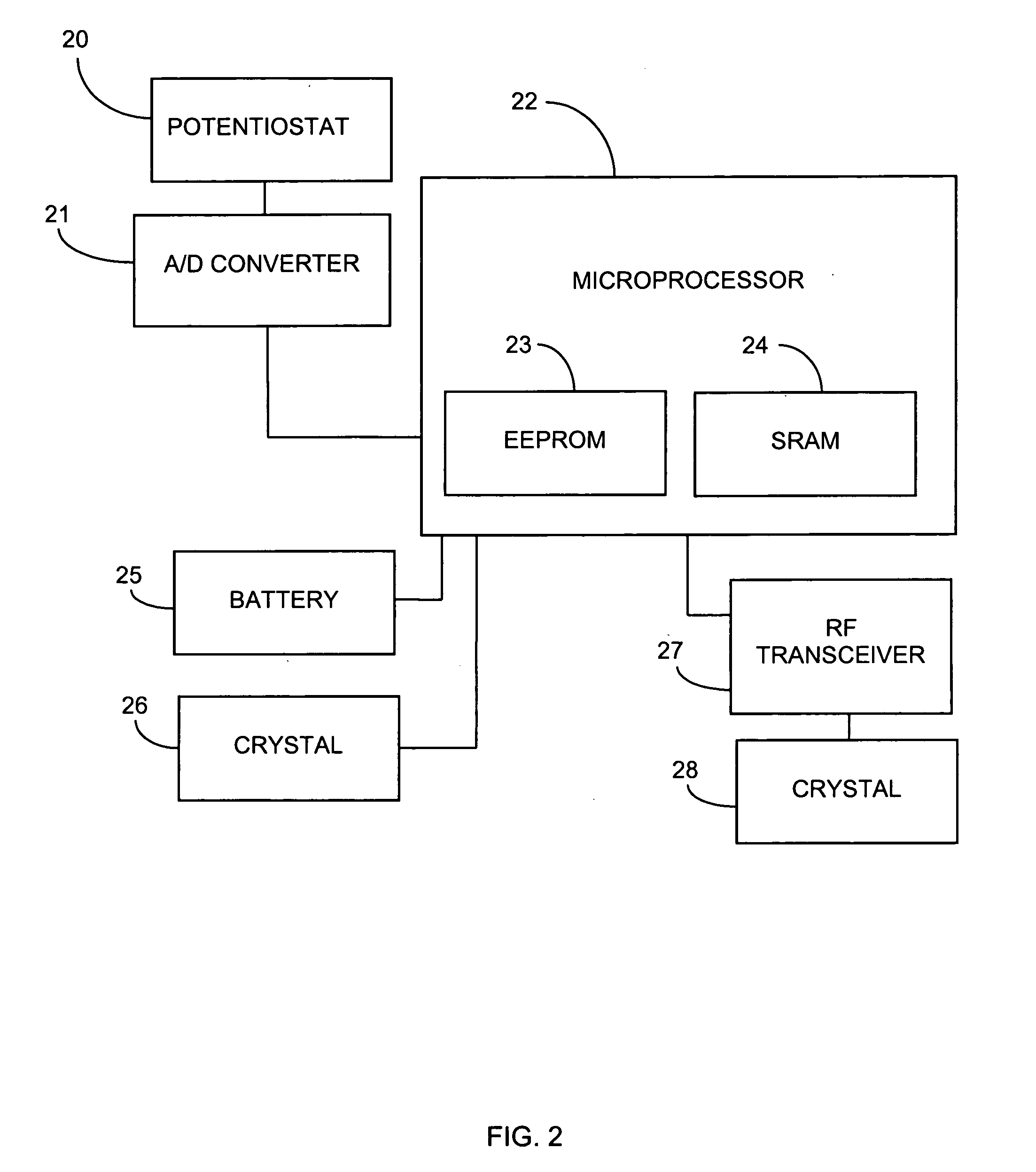
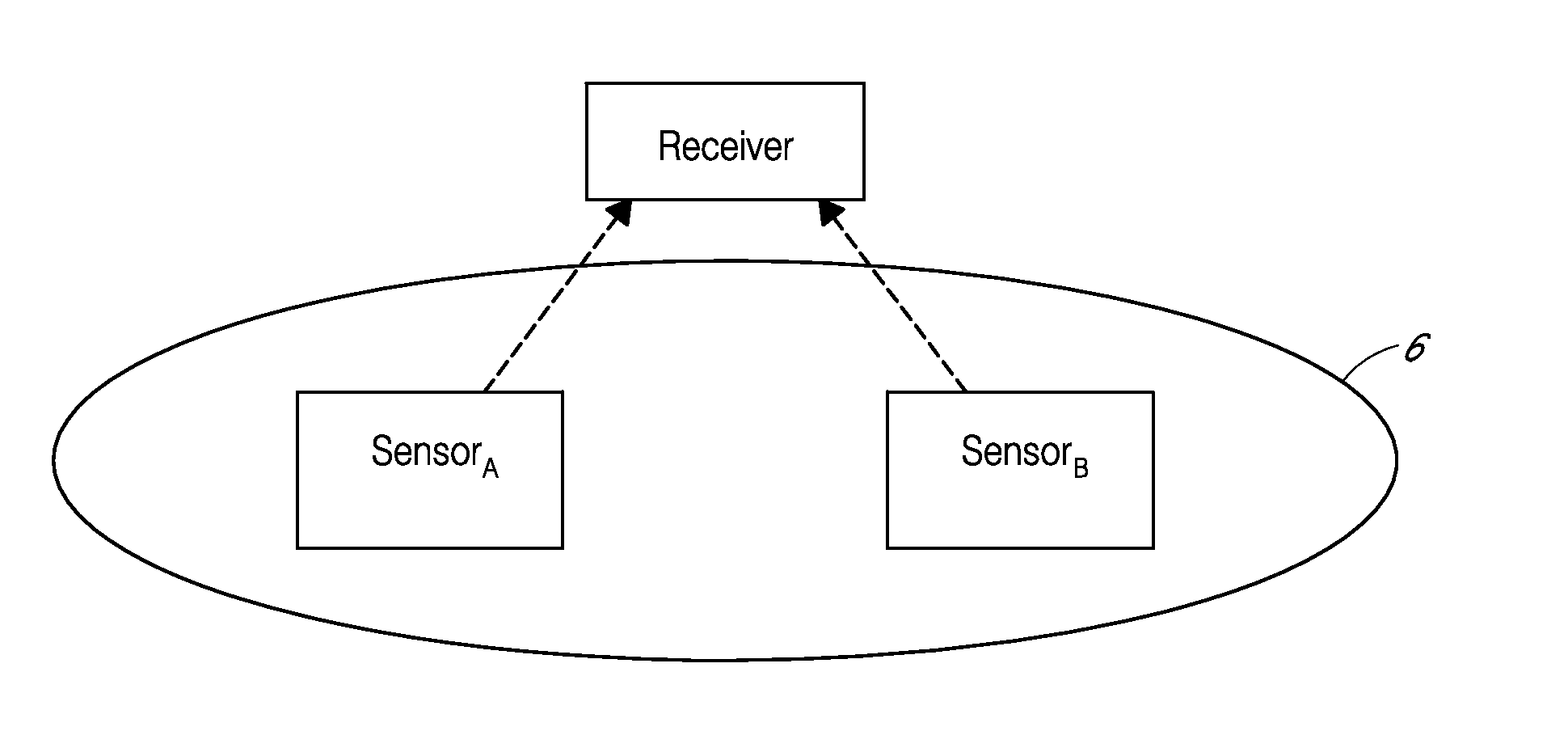
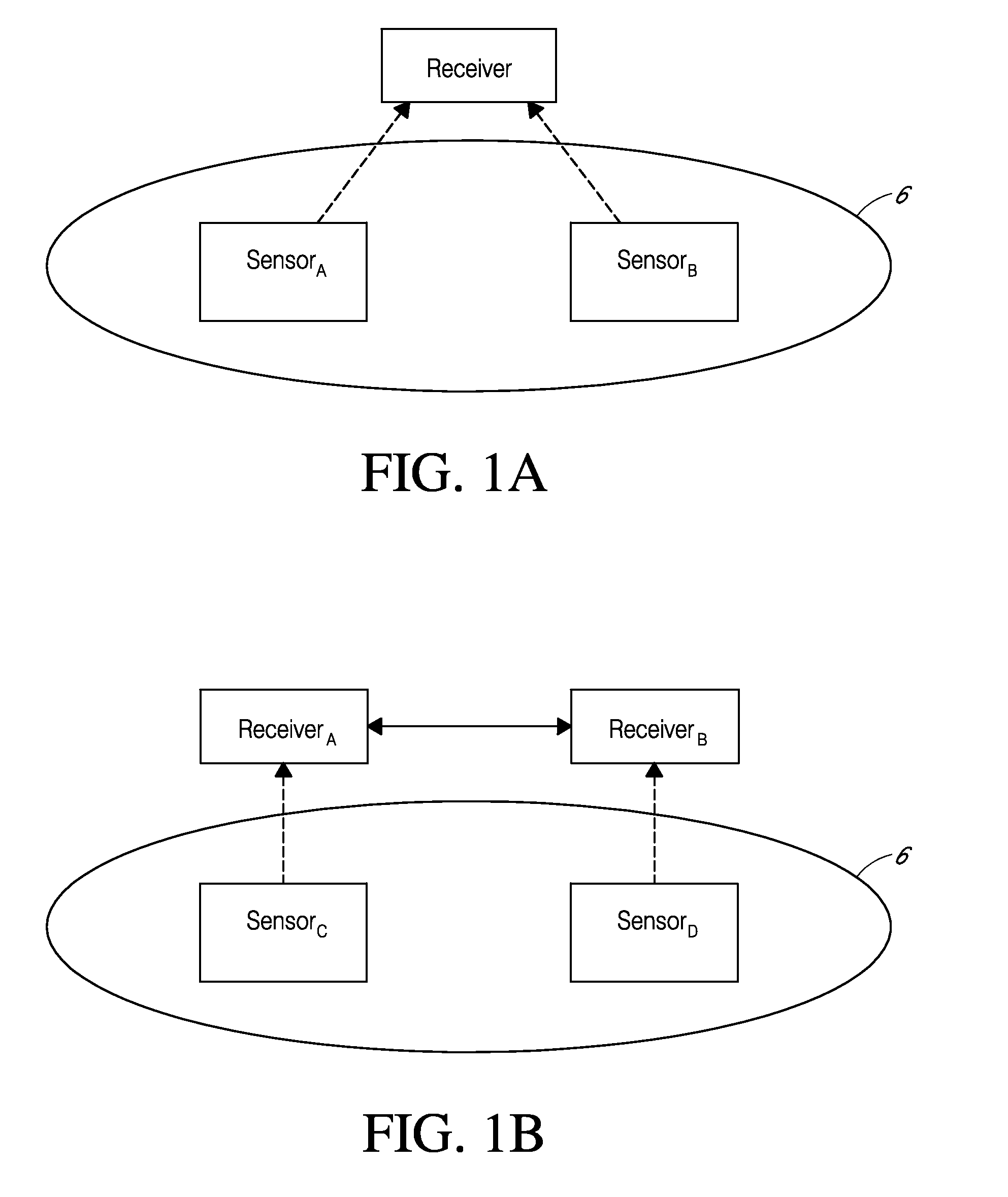
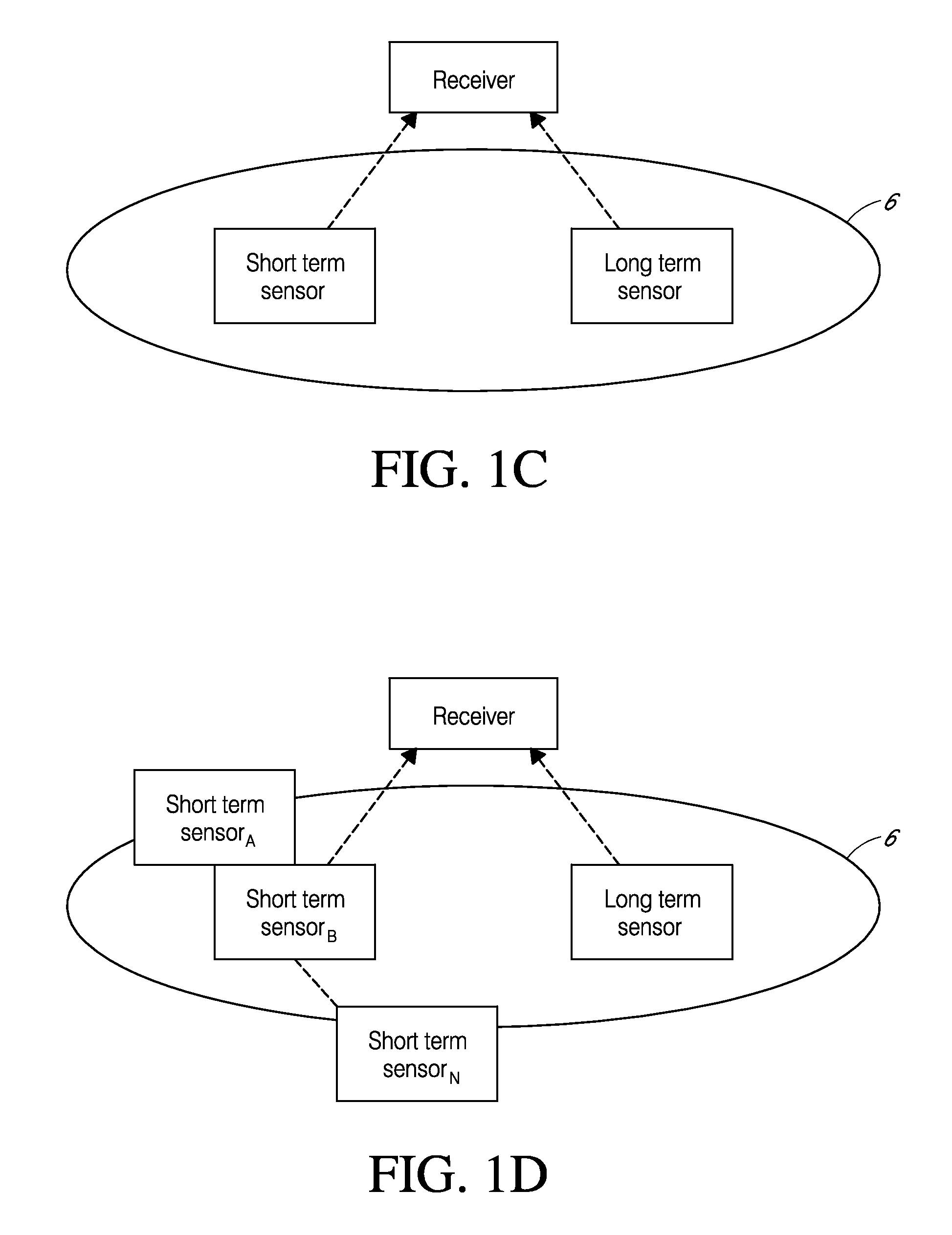
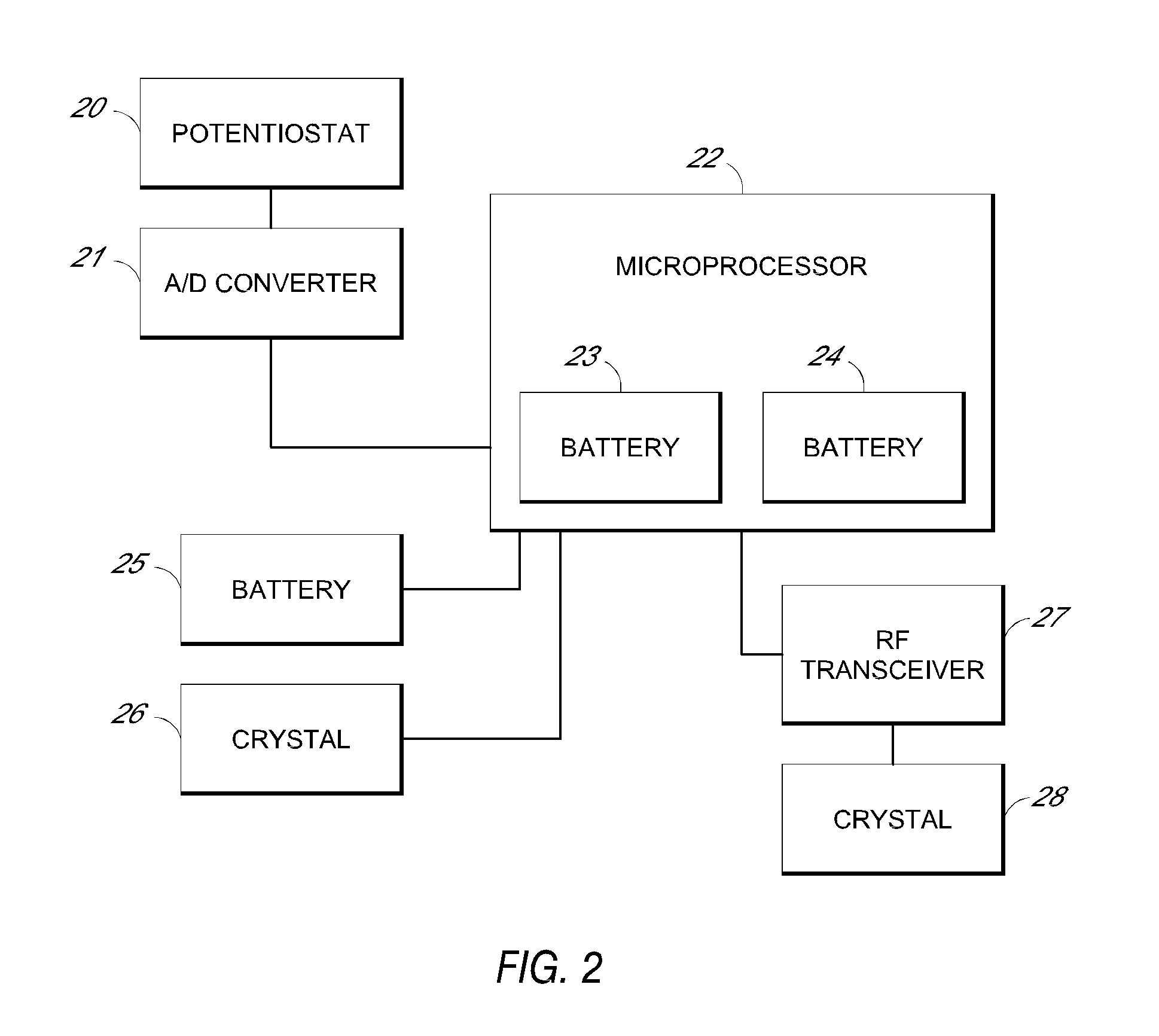
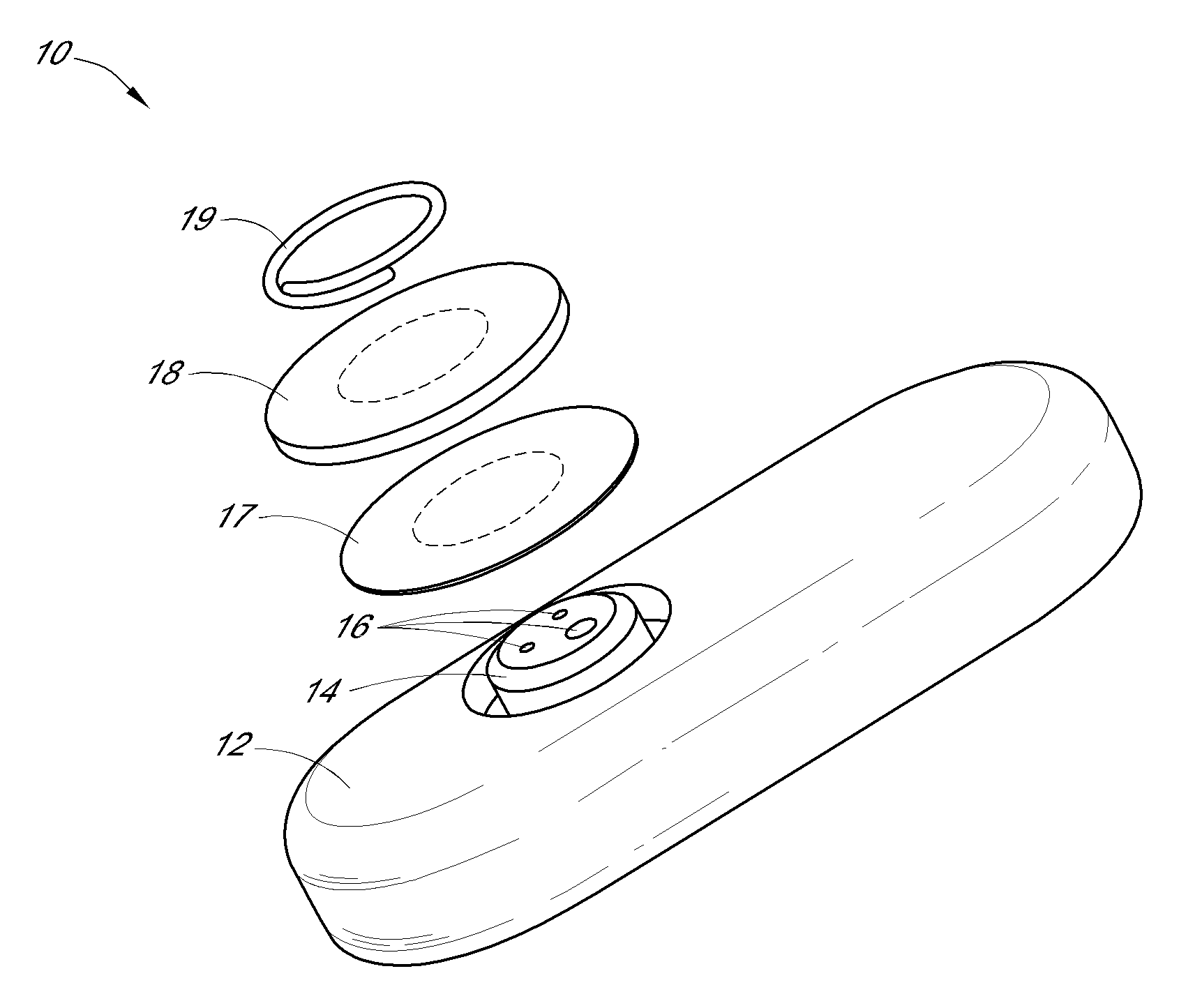
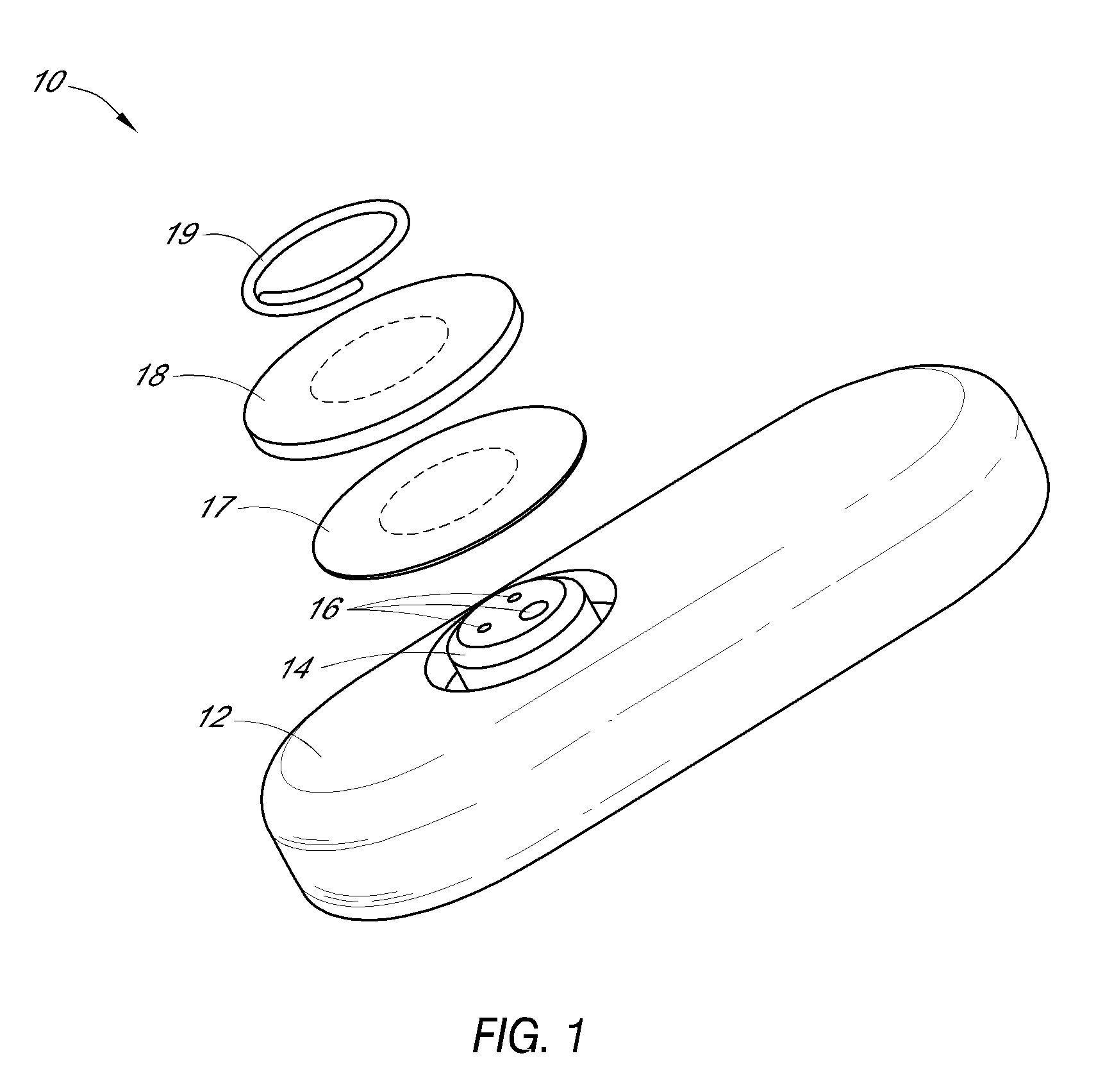
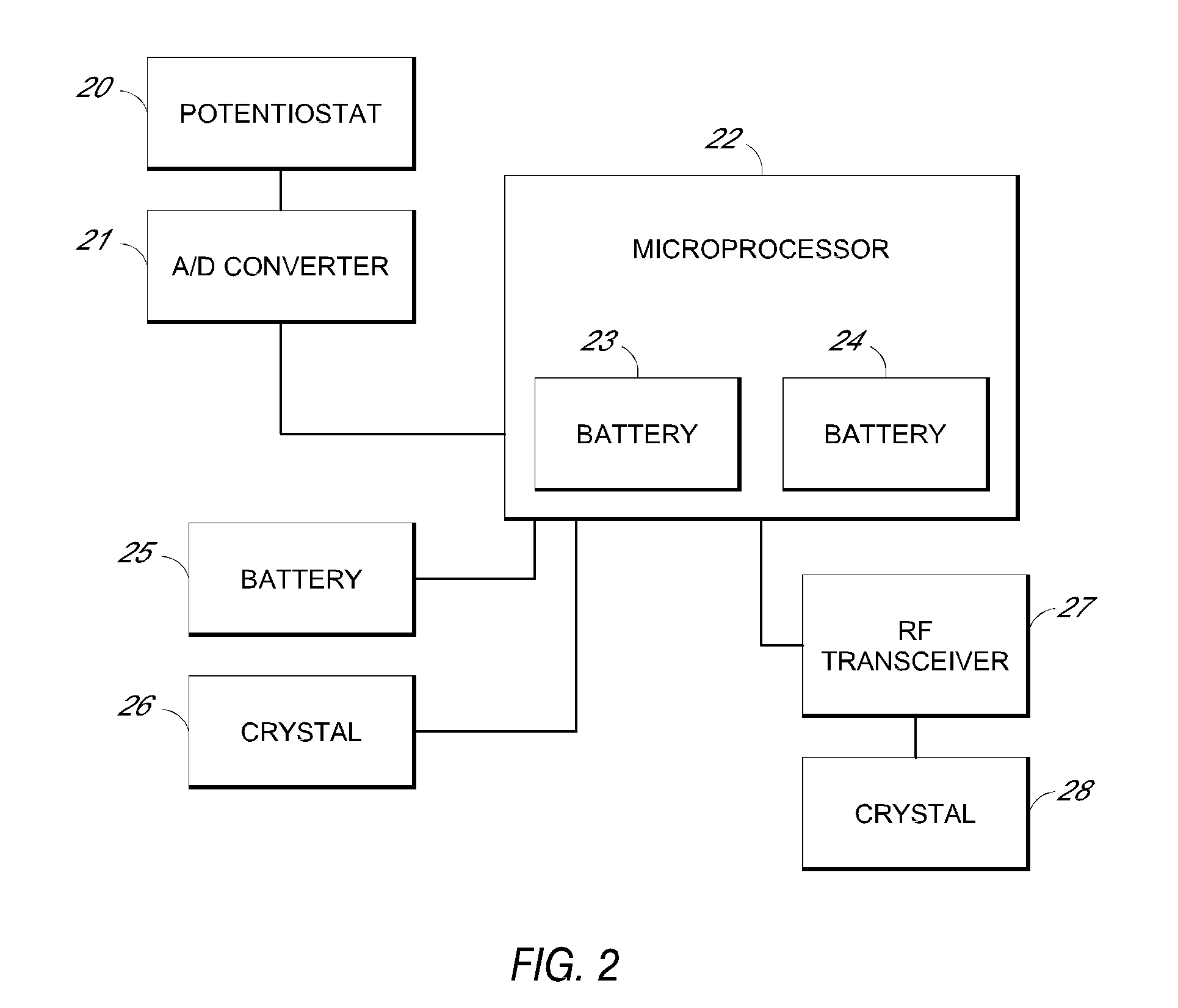
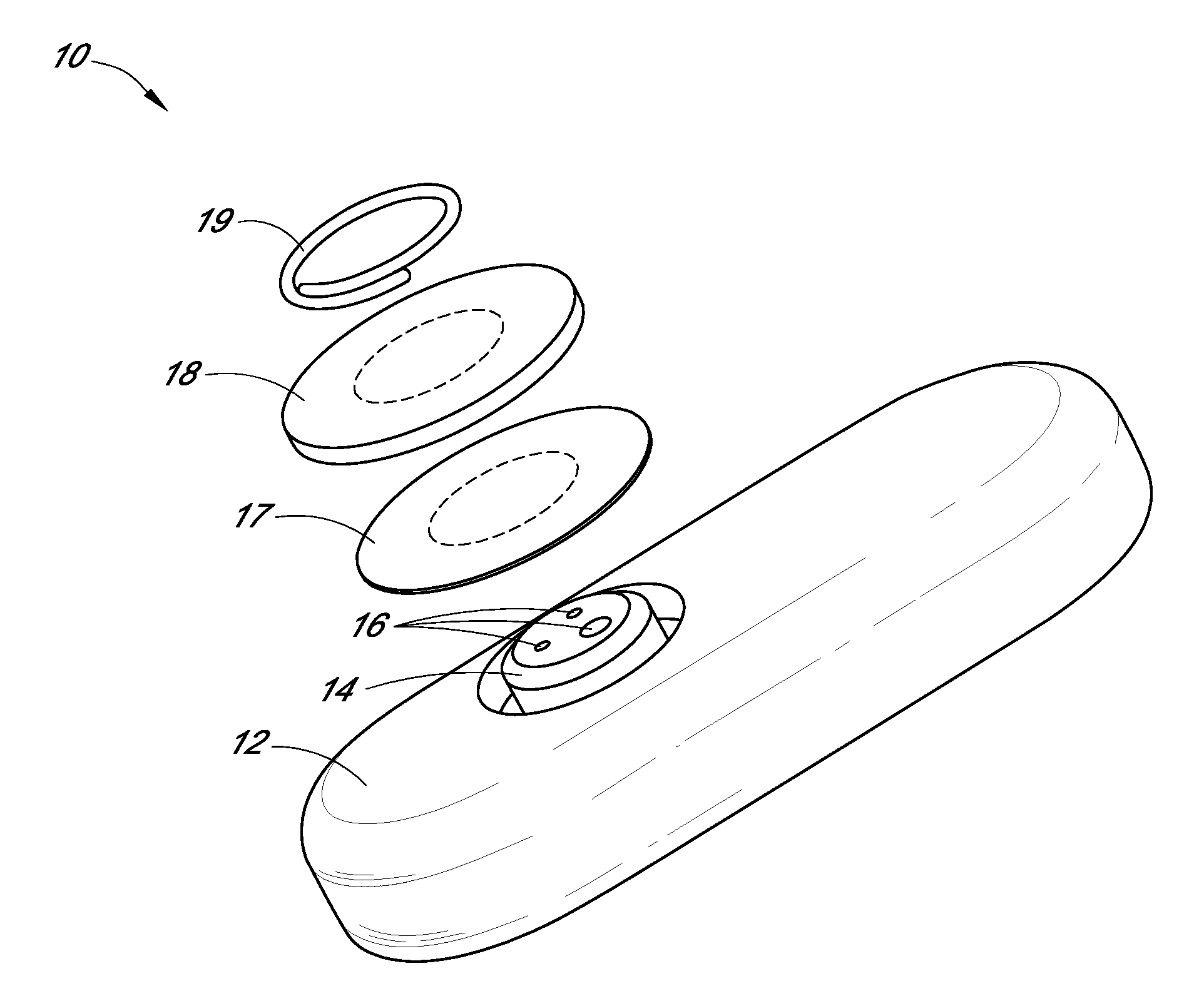
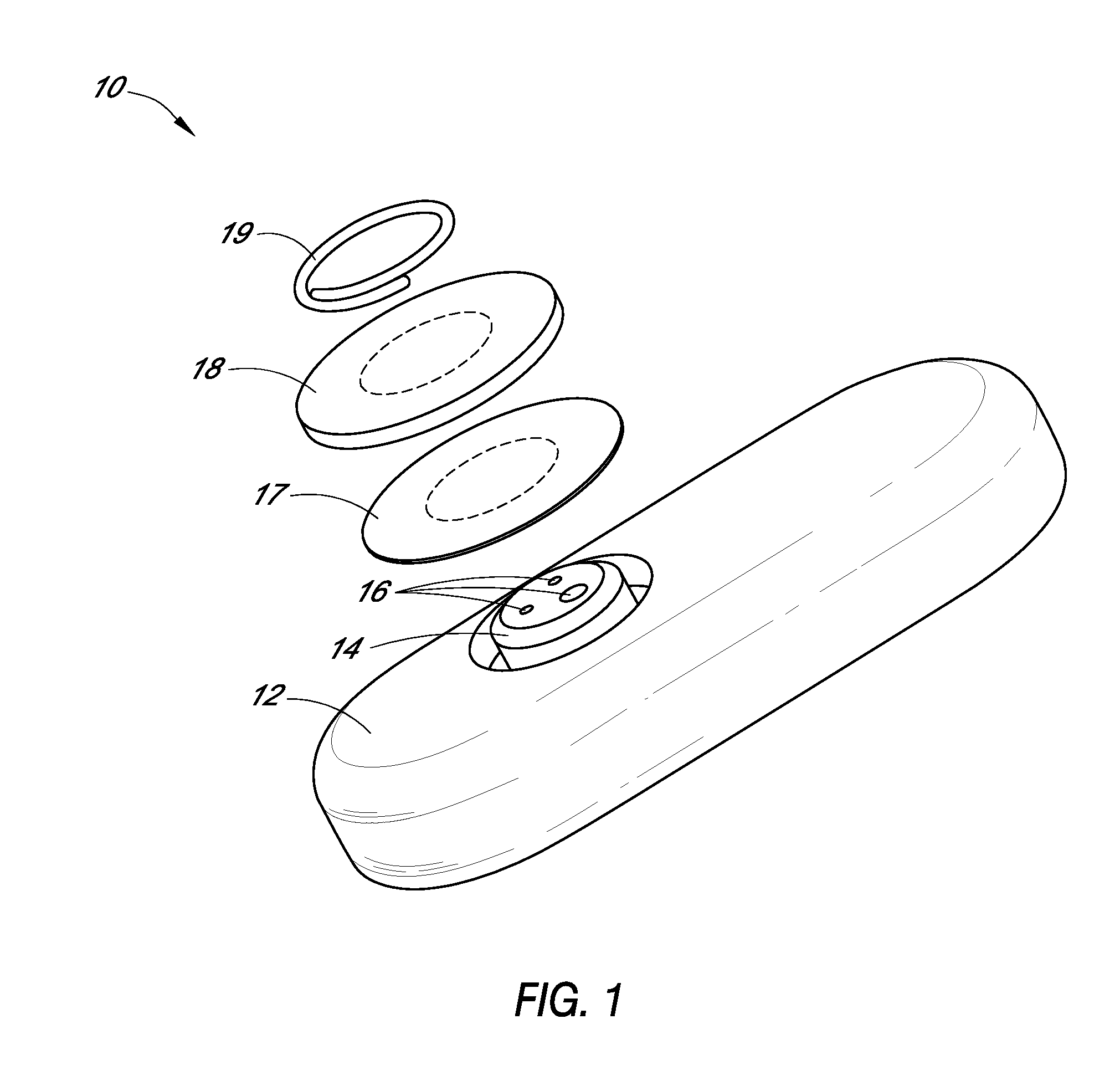
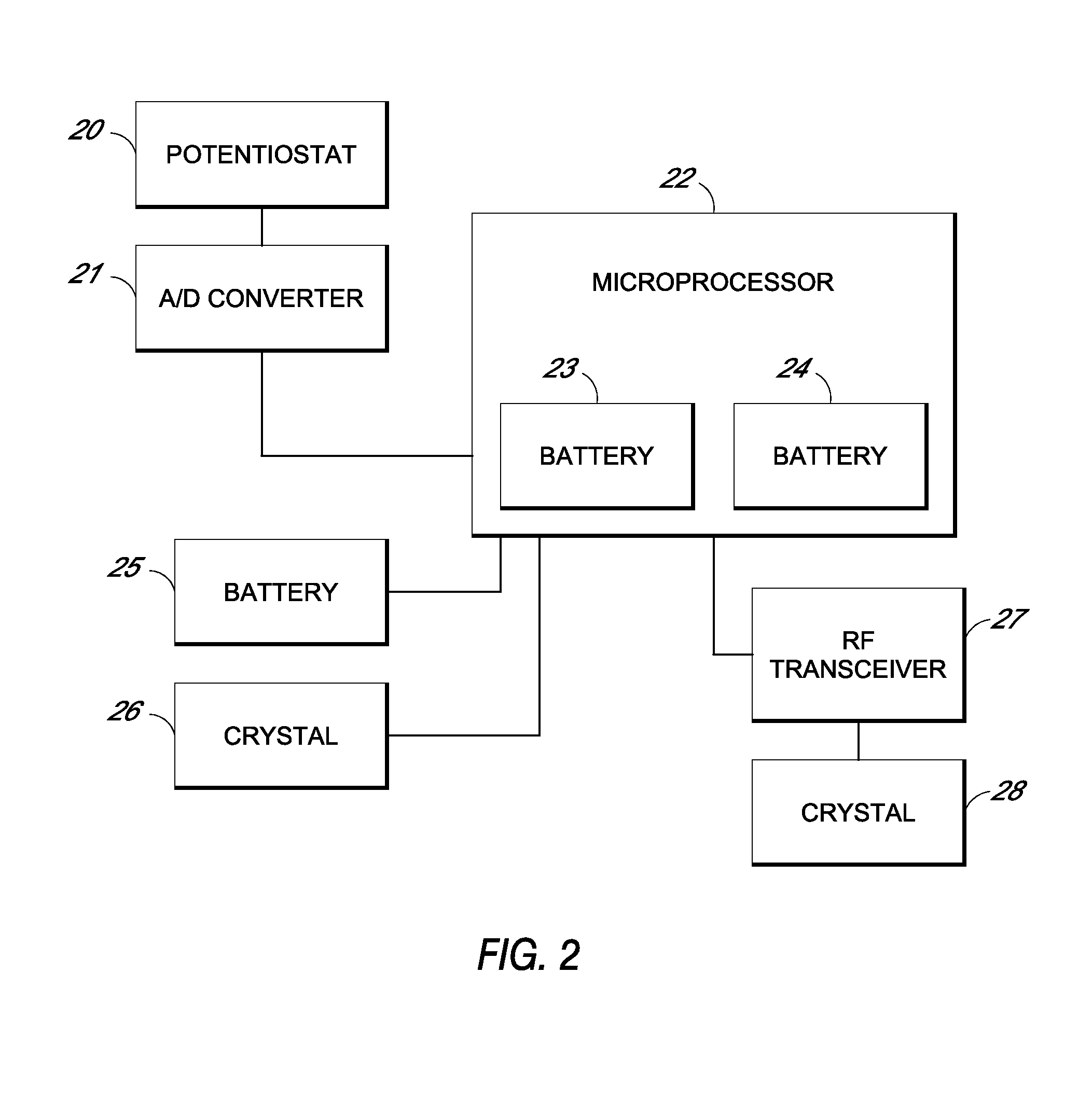
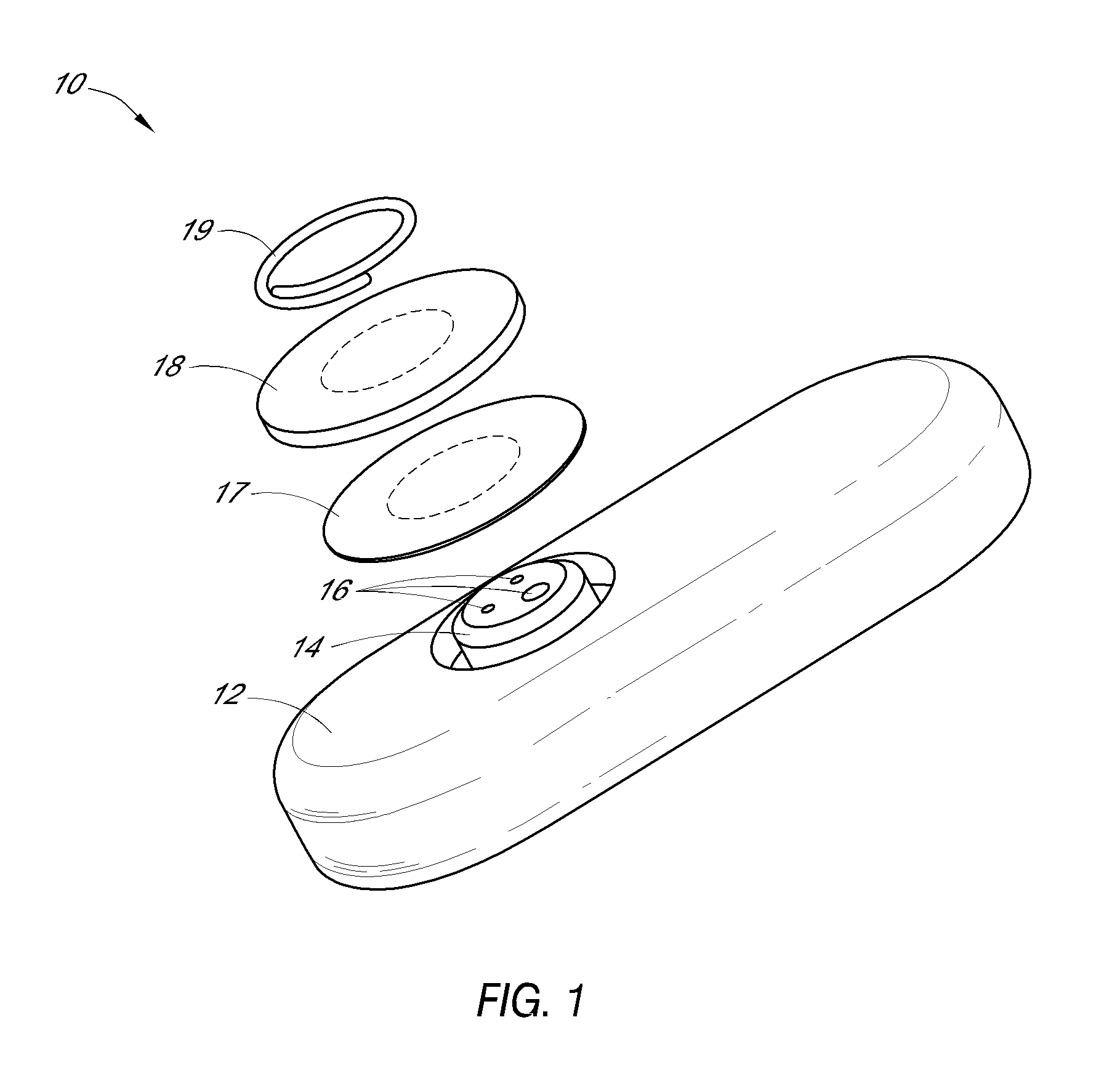
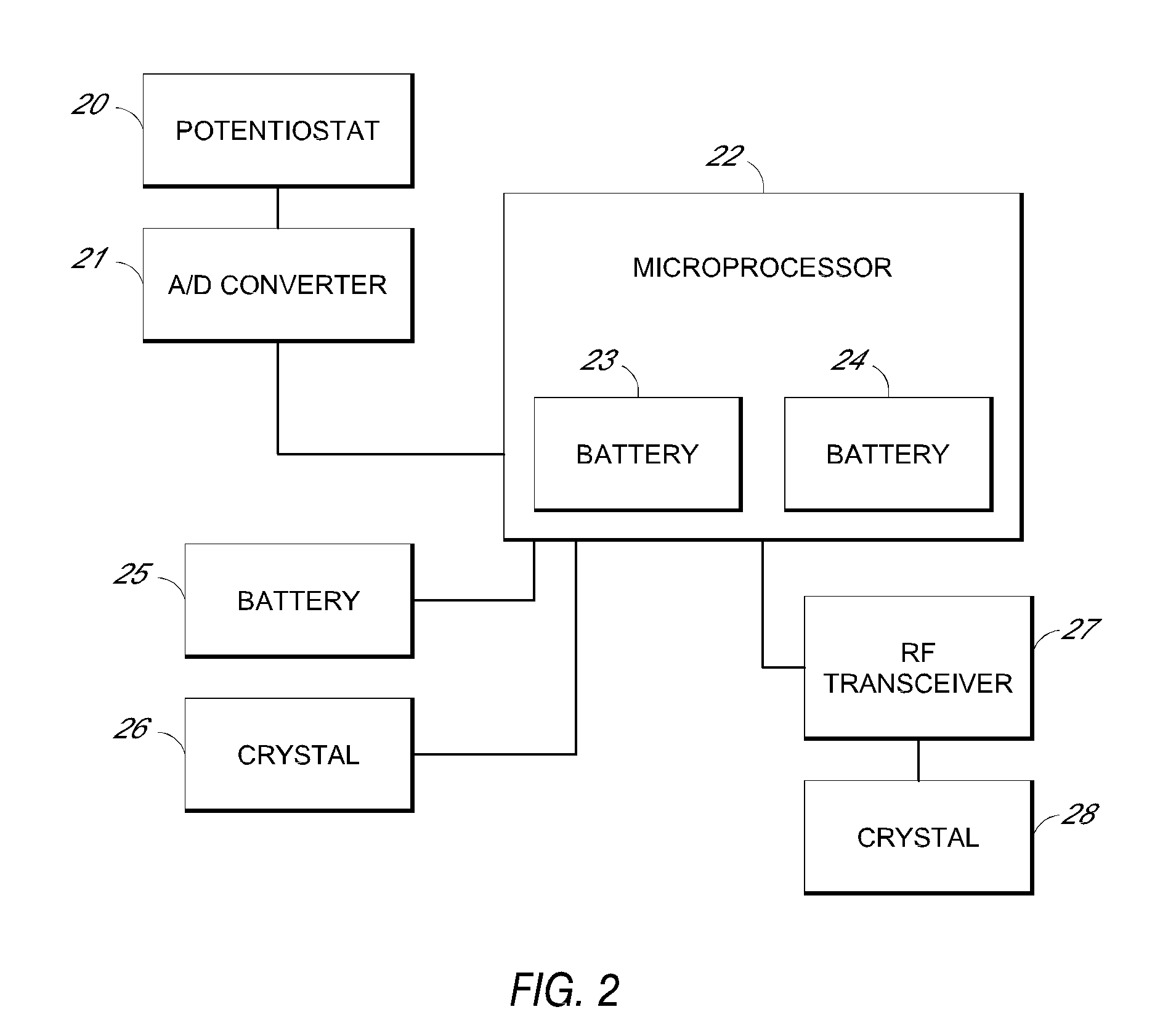
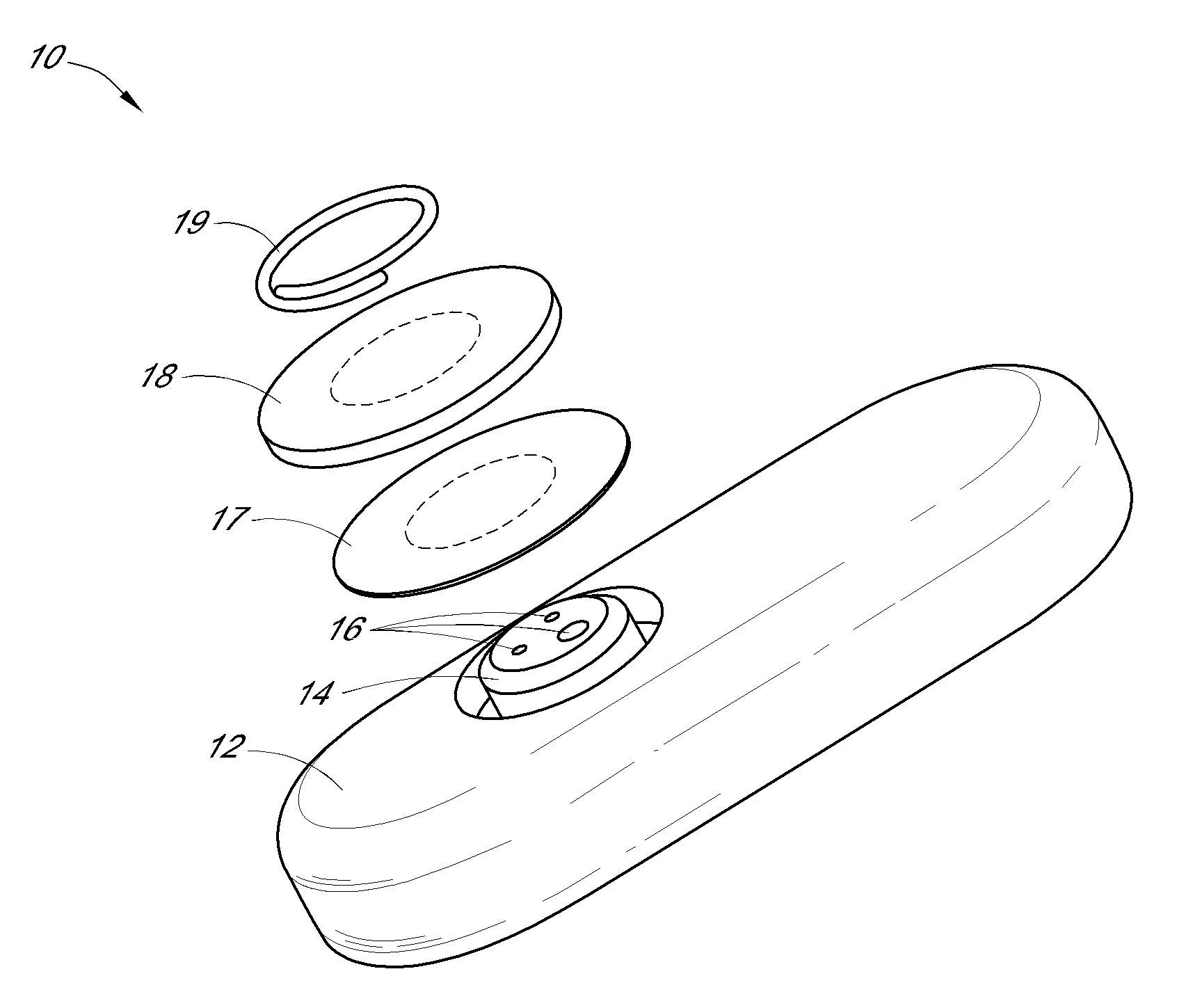
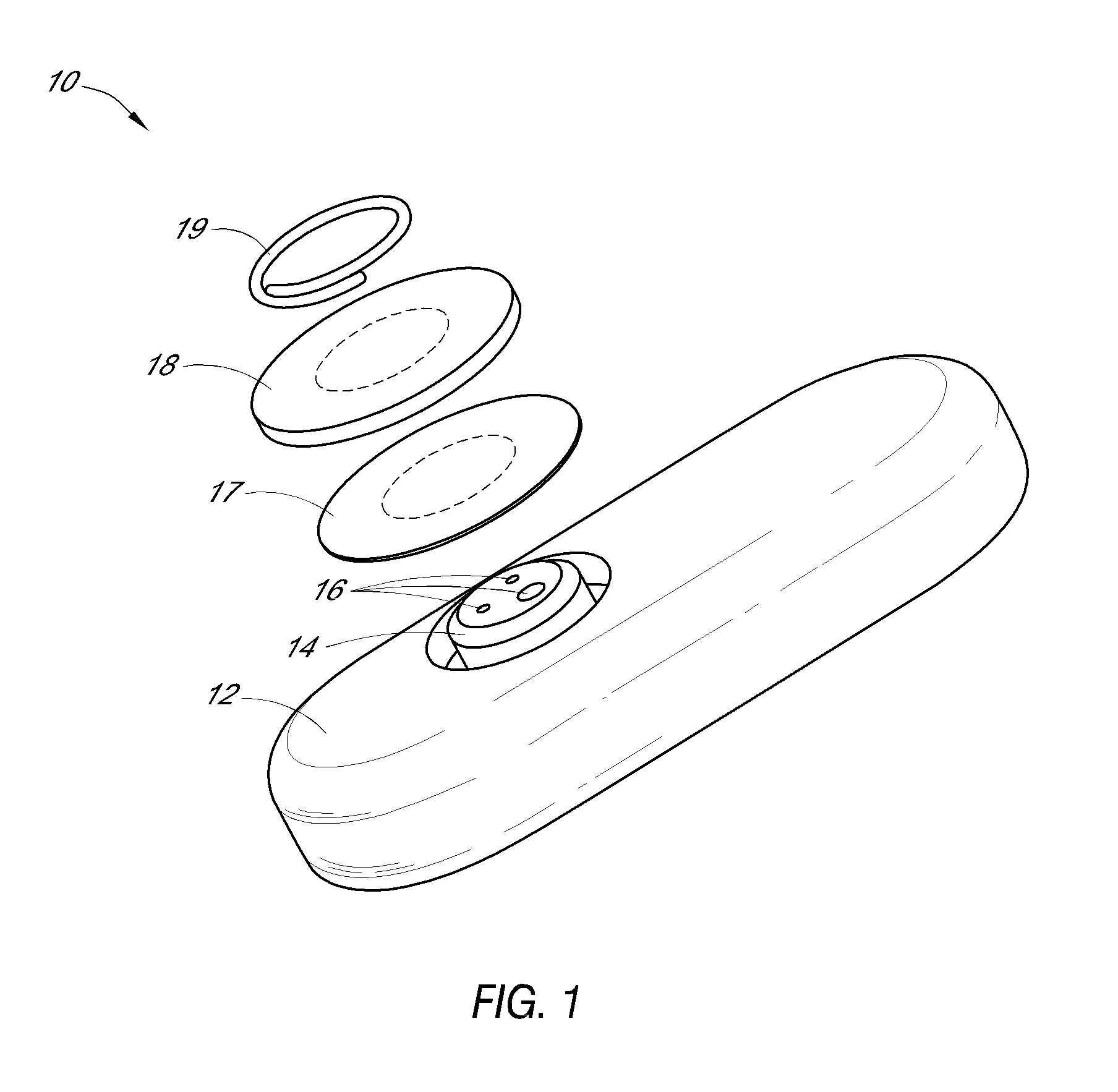
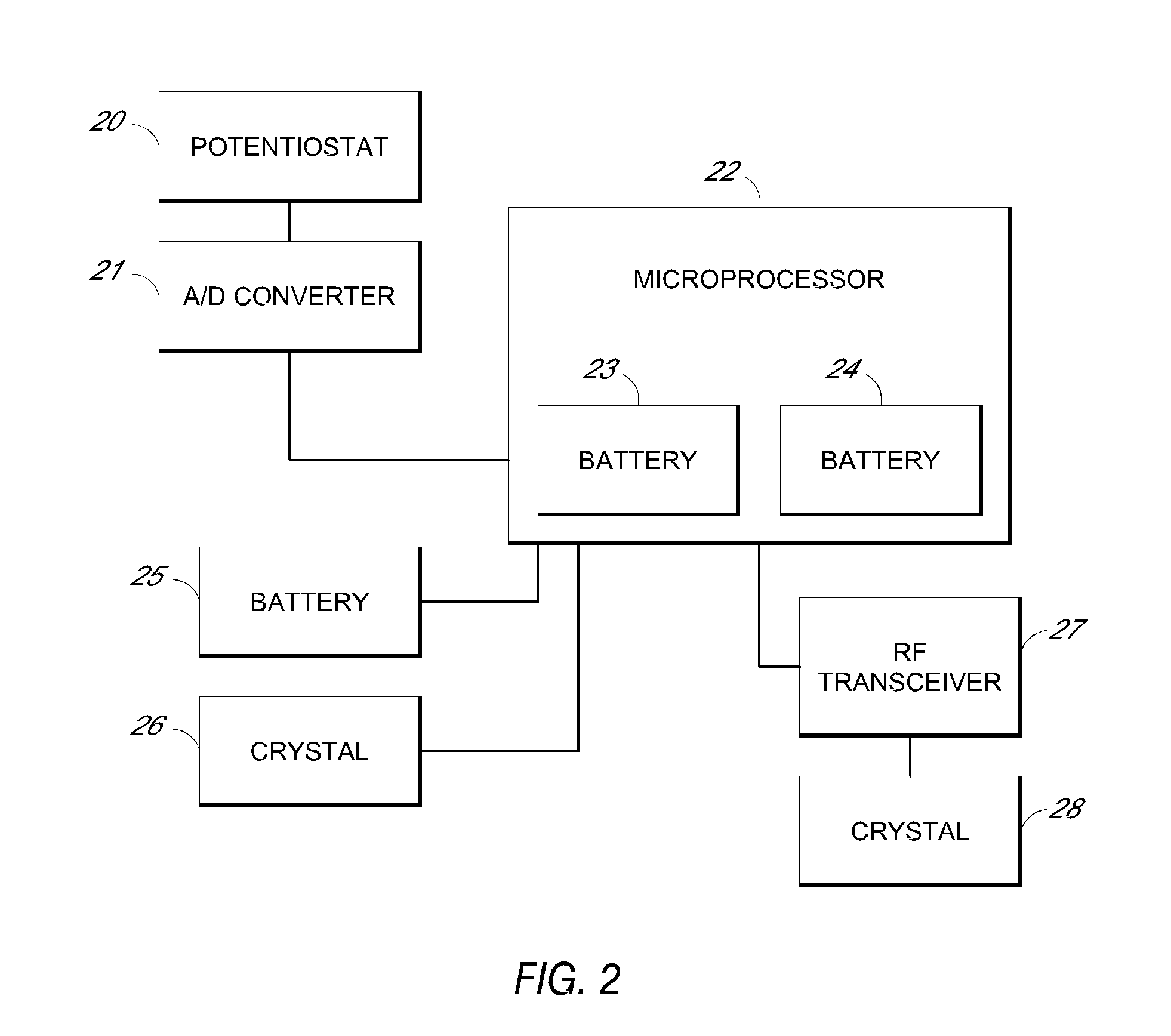
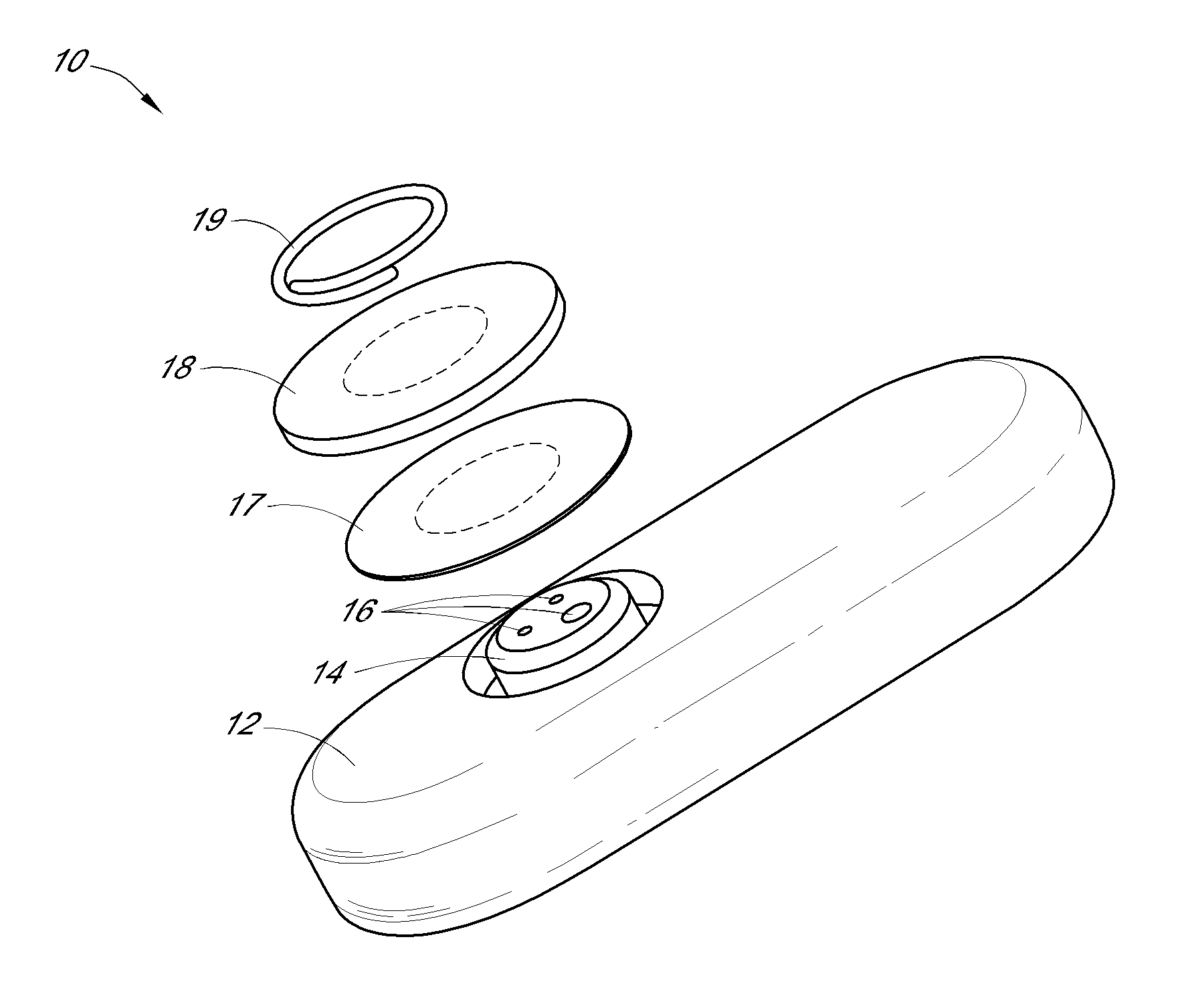
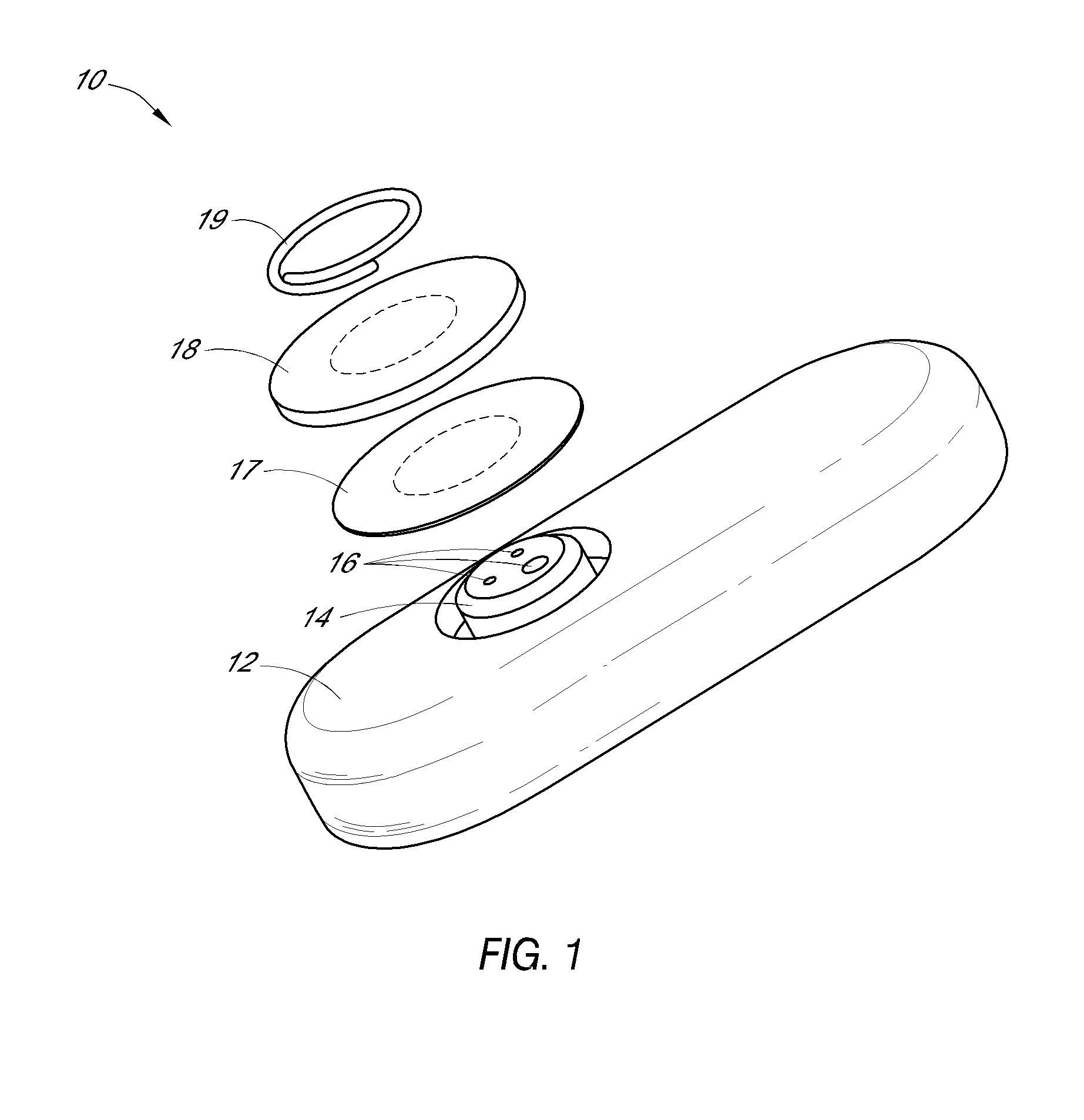
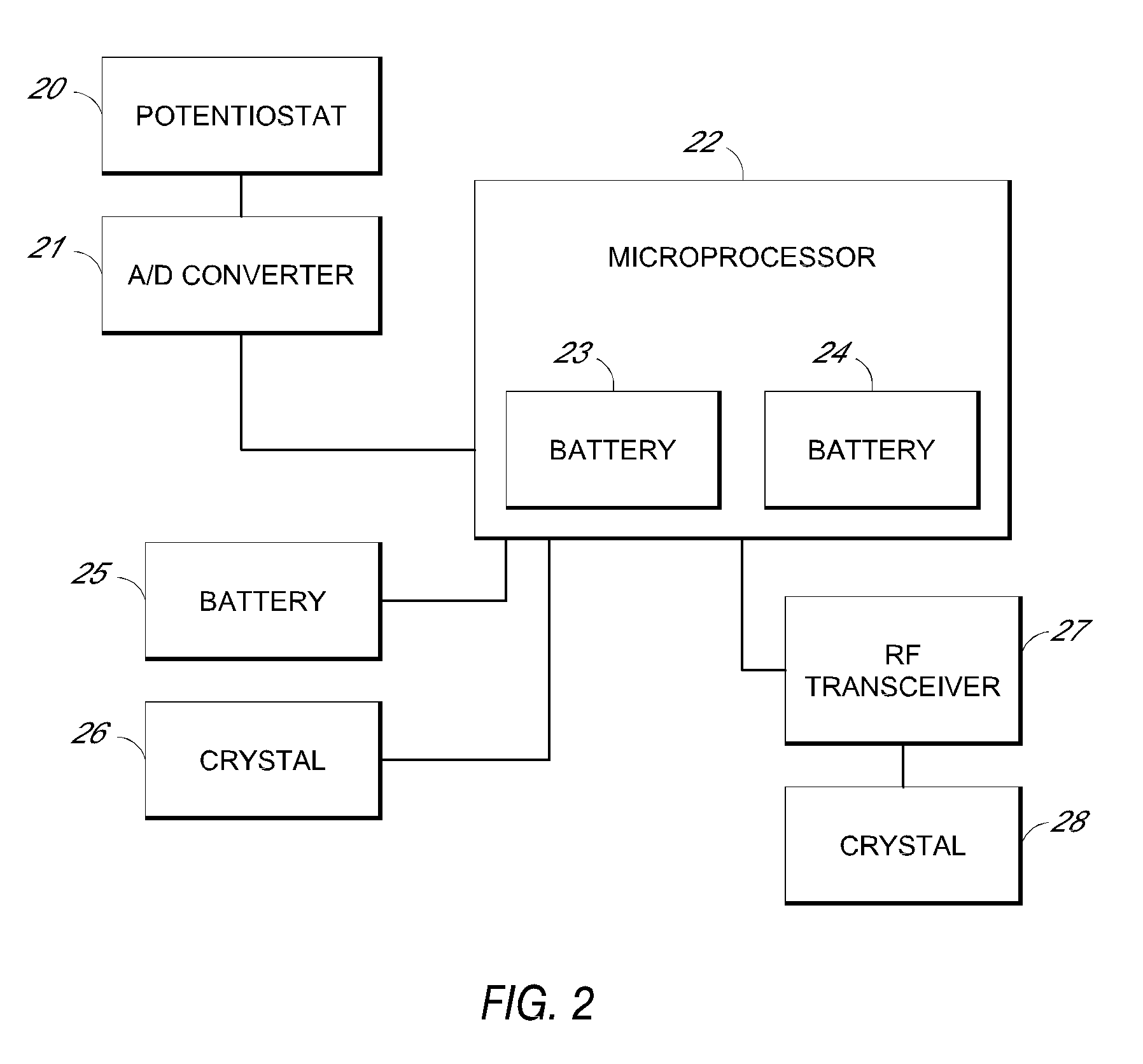
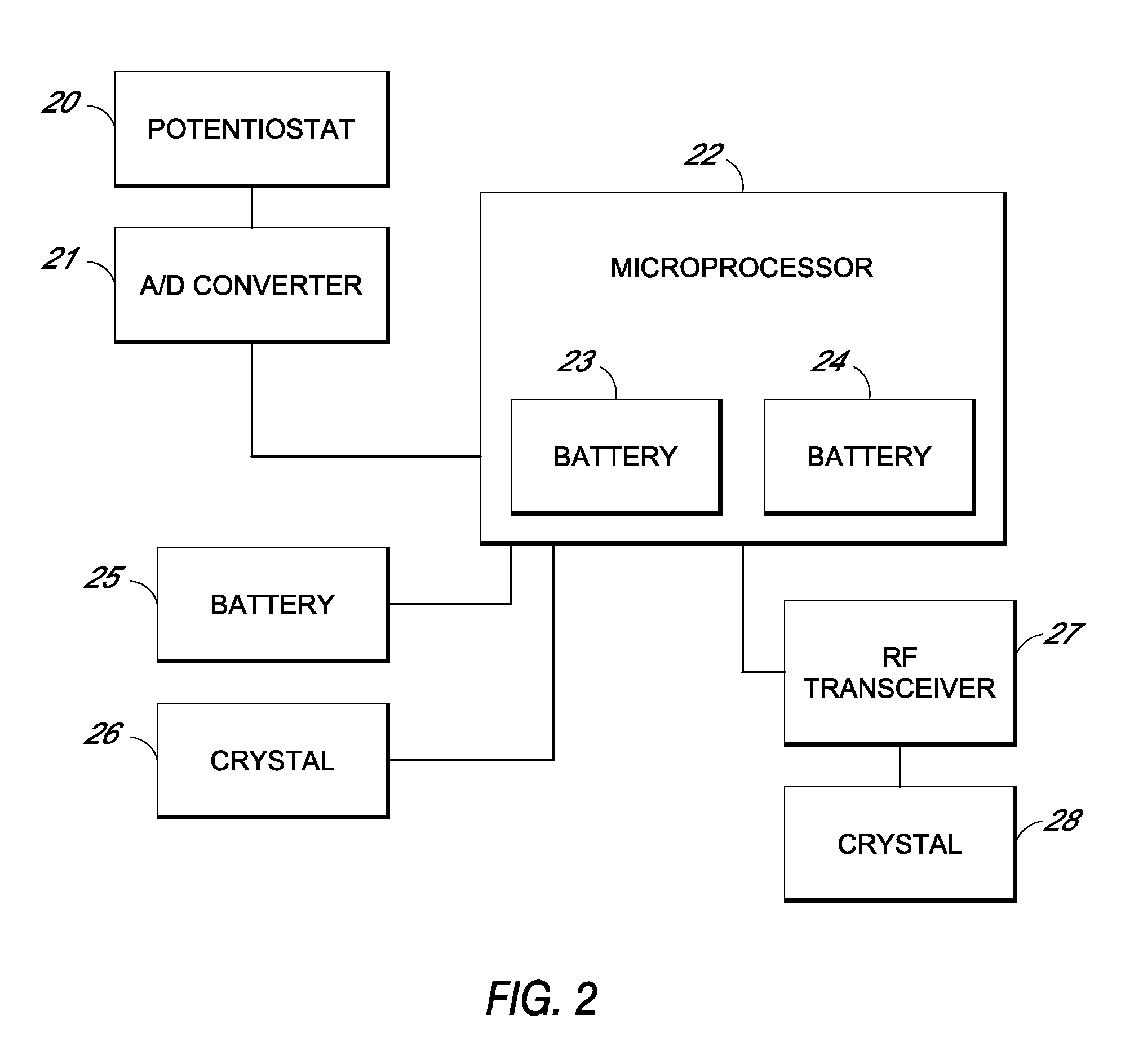
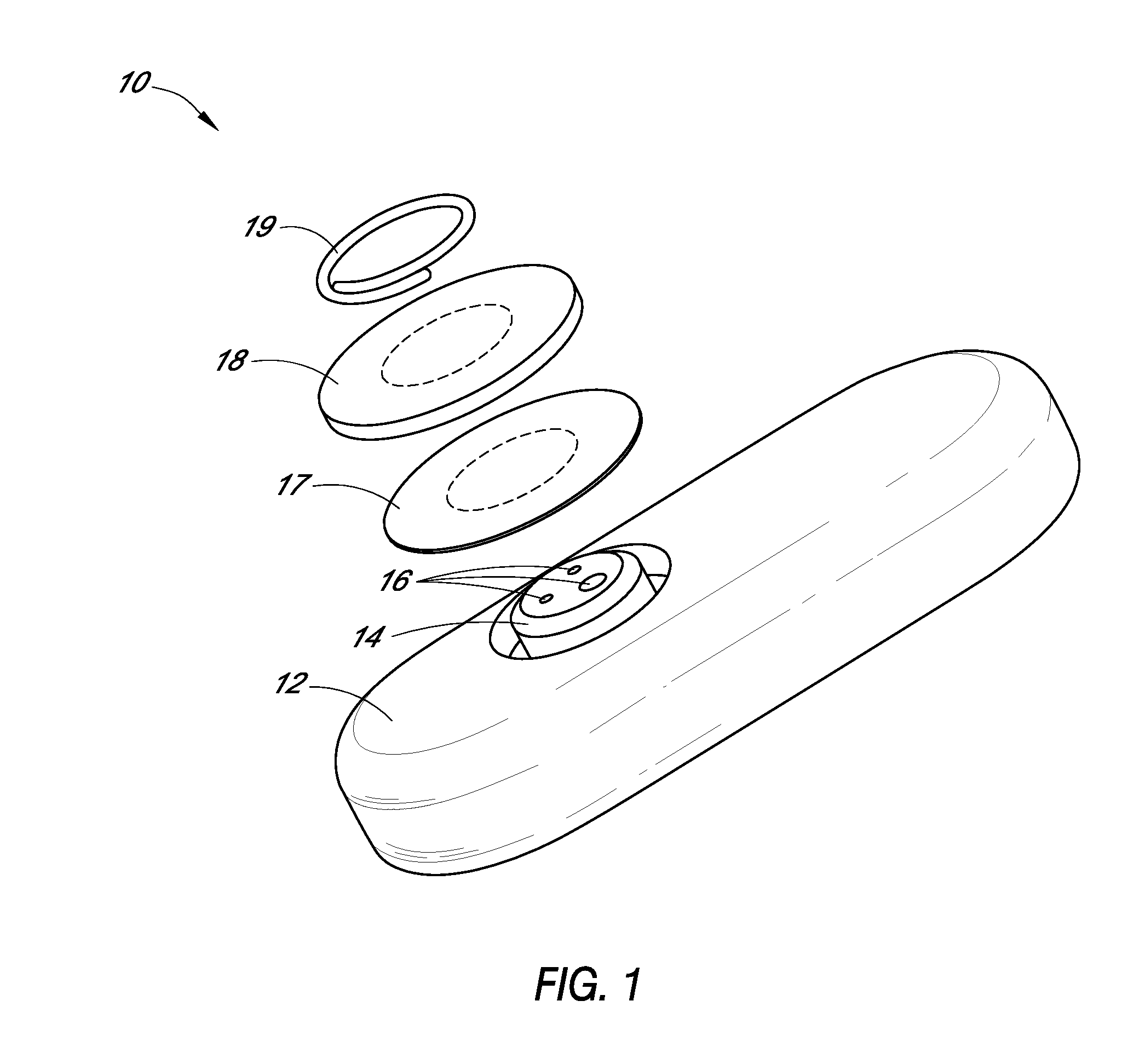
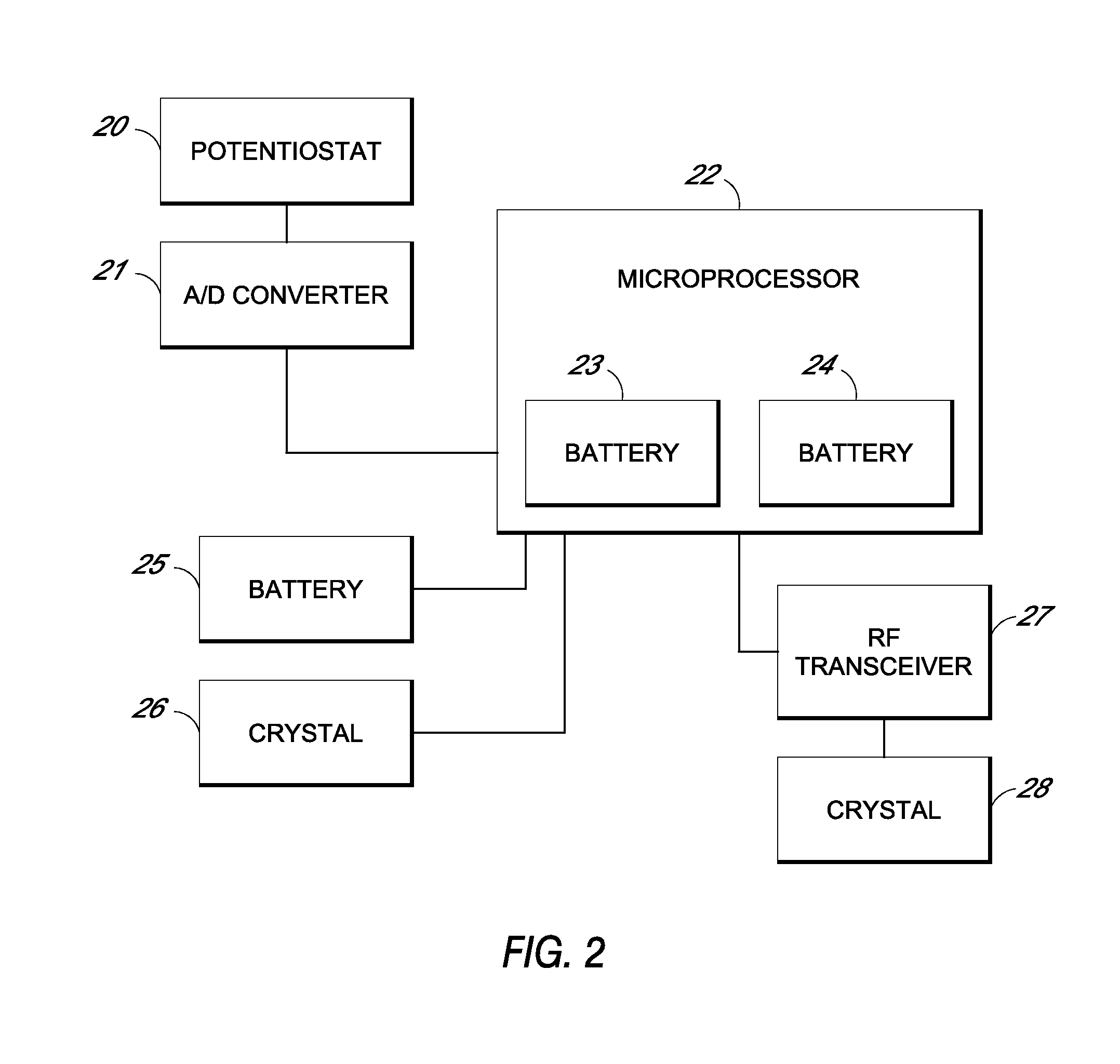
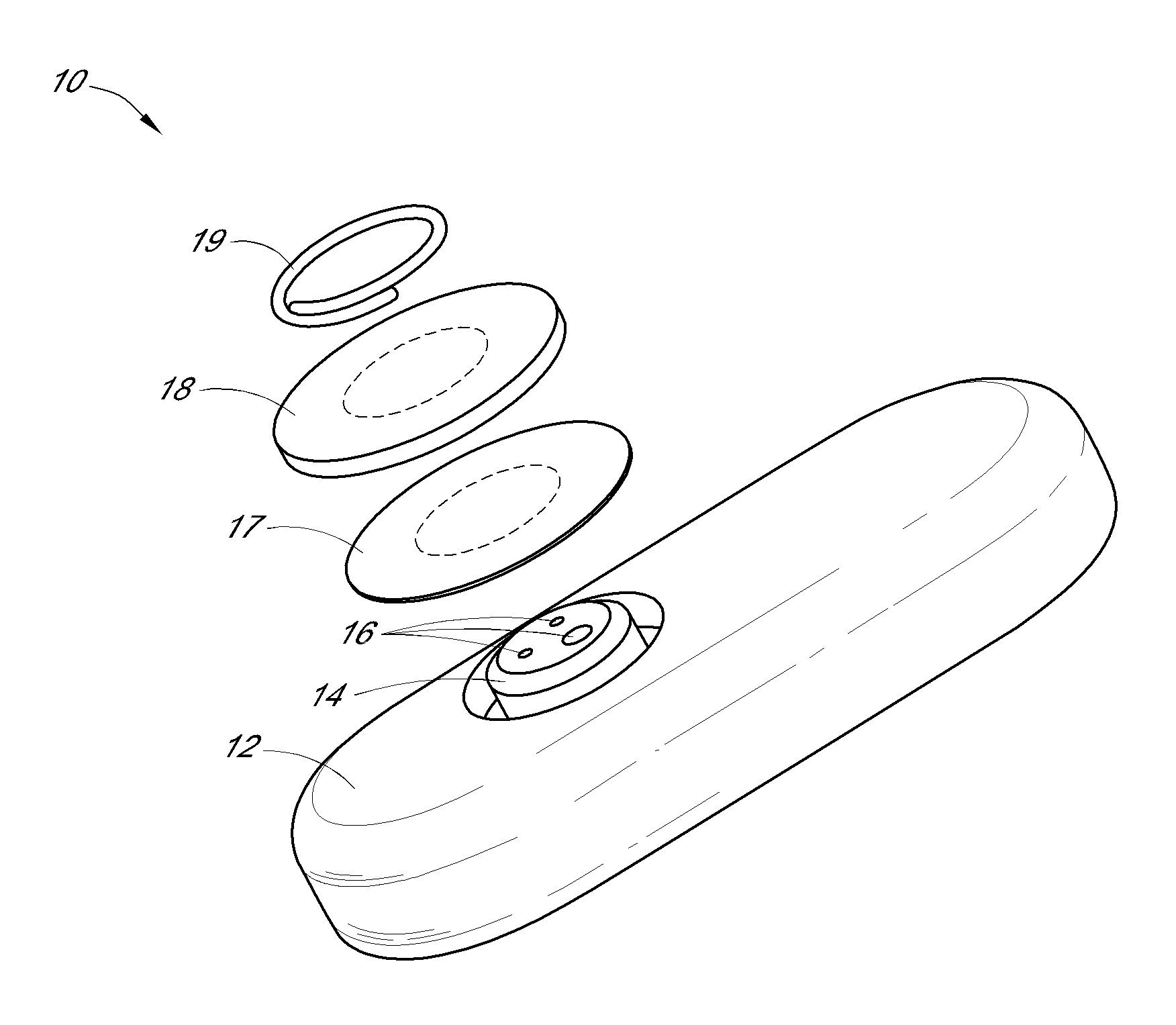
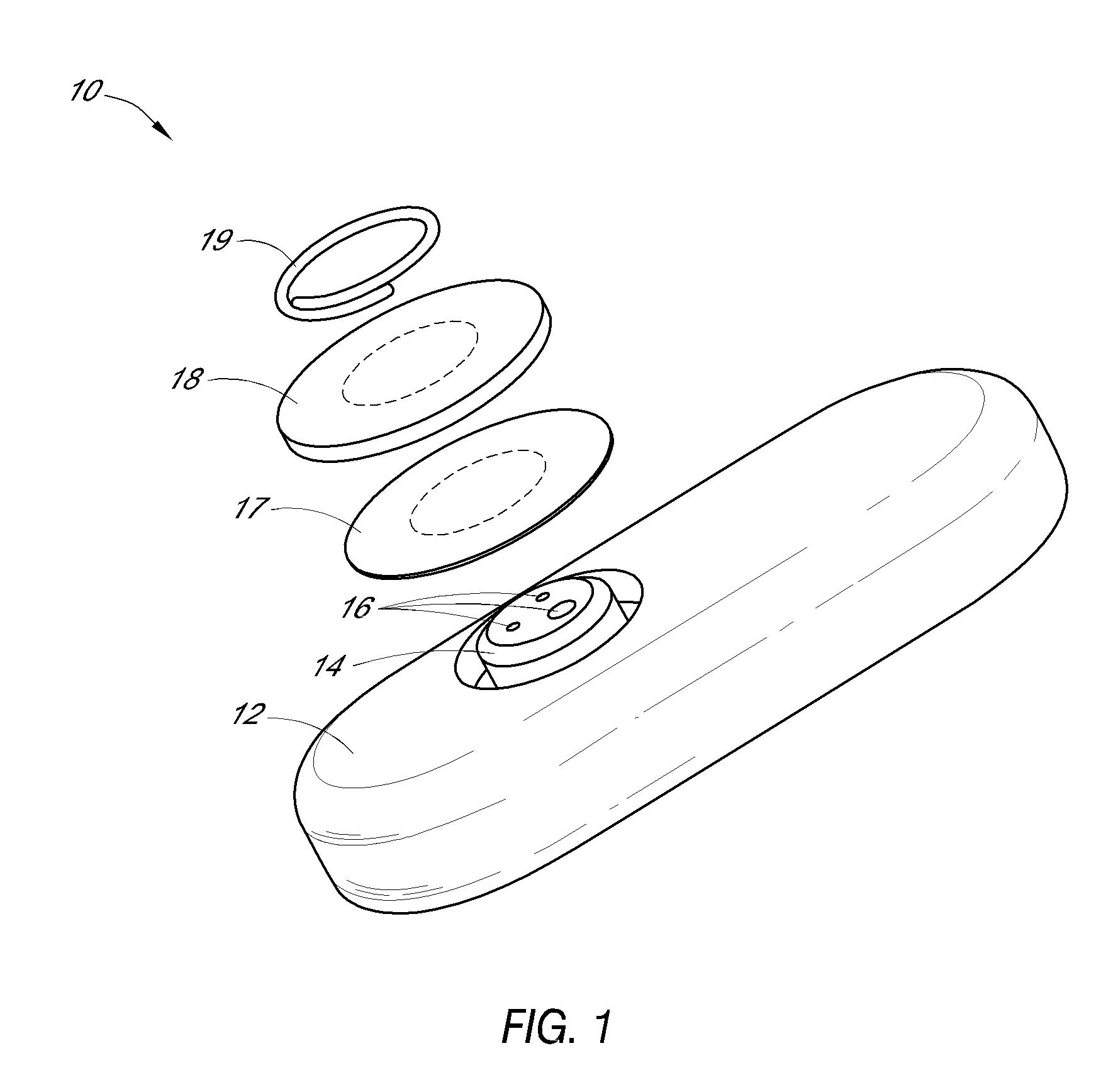
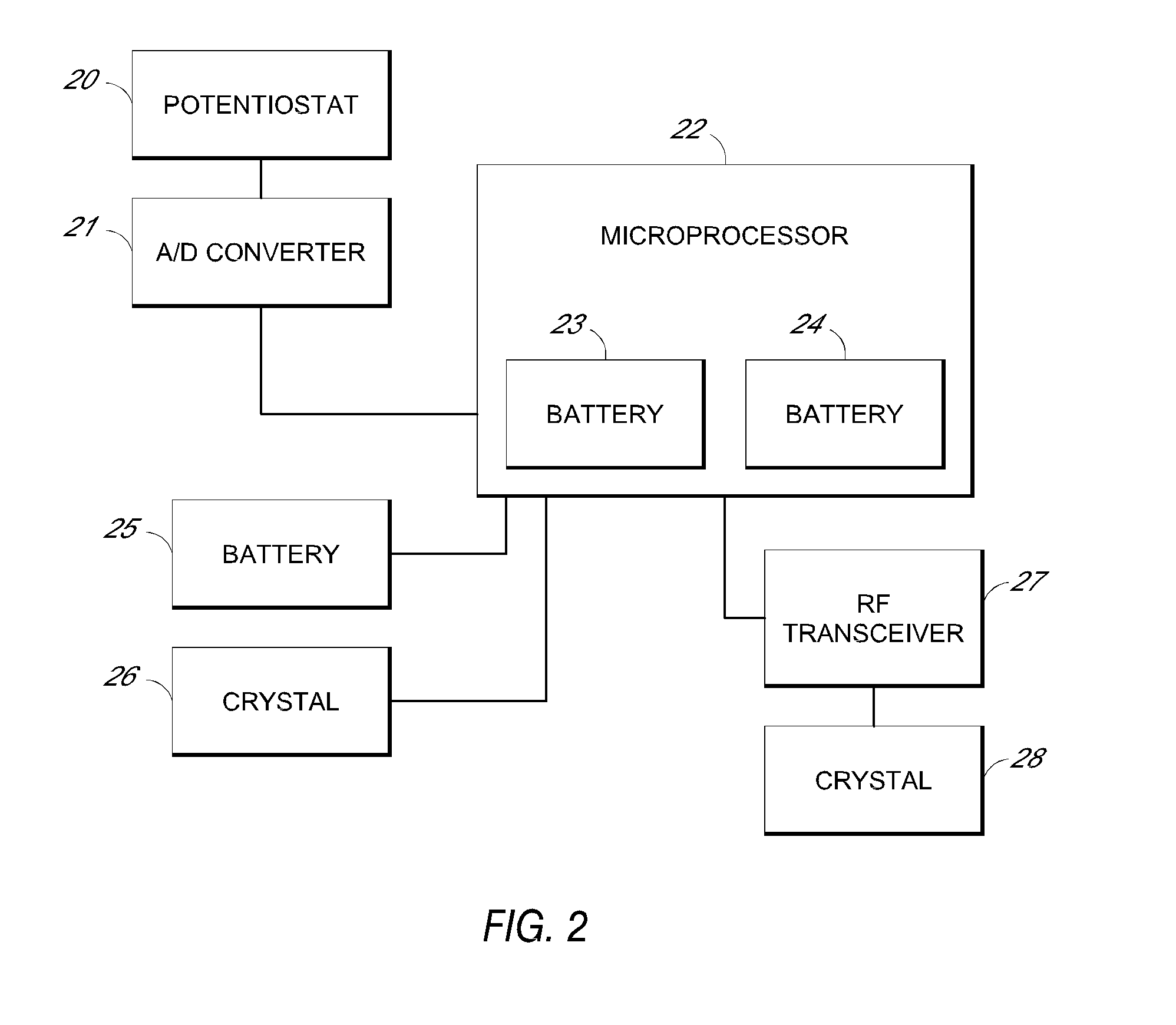
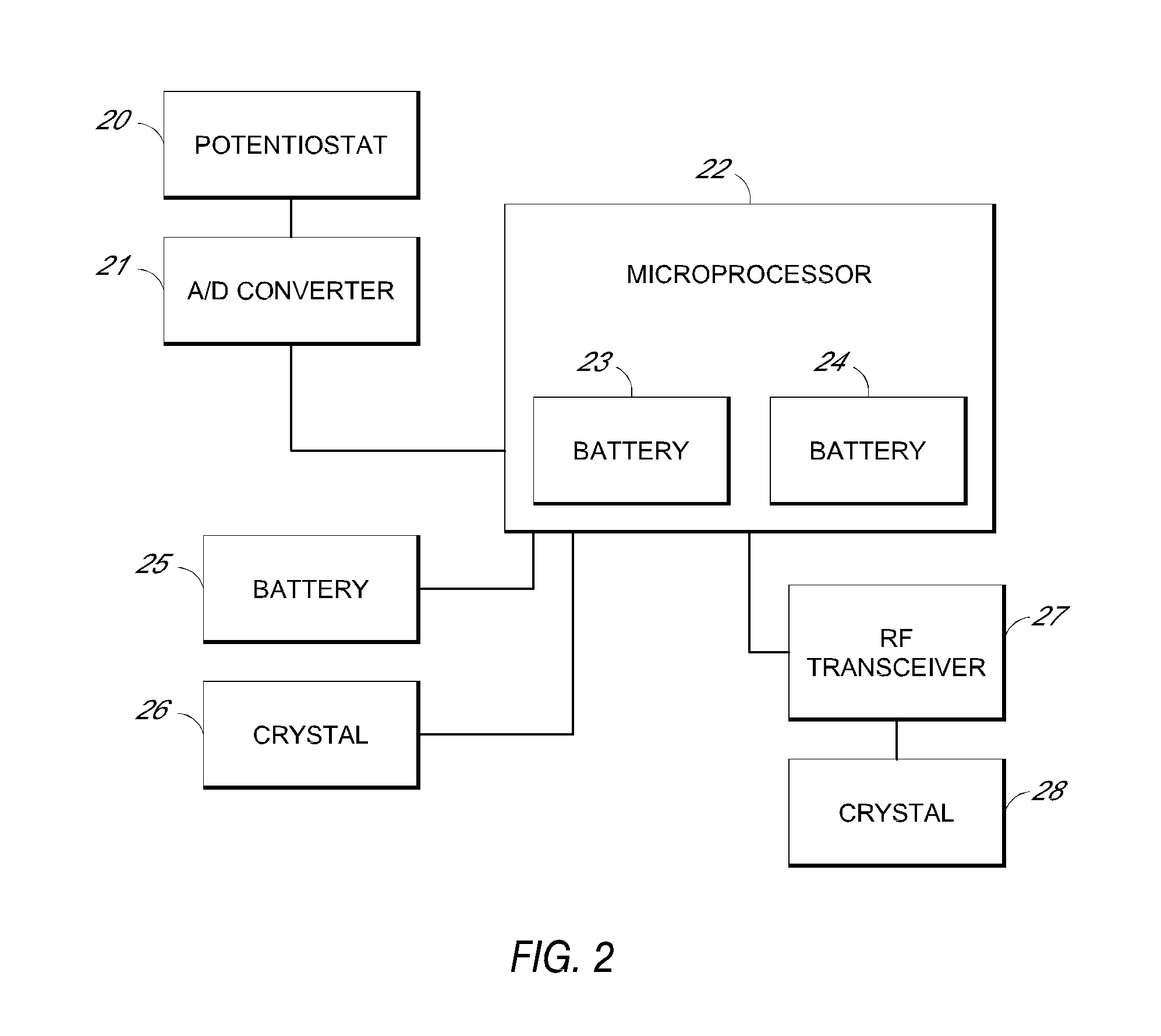
System and methods for processing analyte sensor data
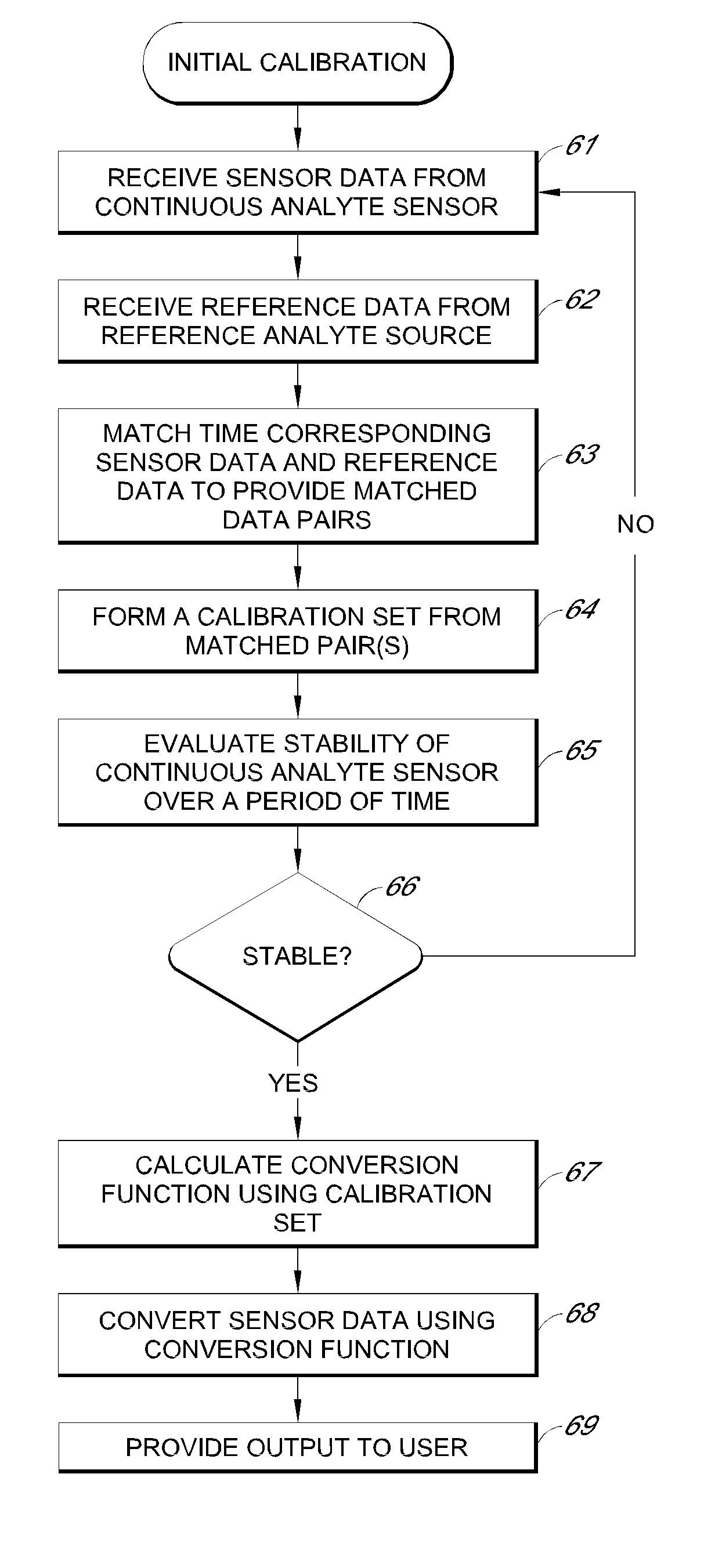
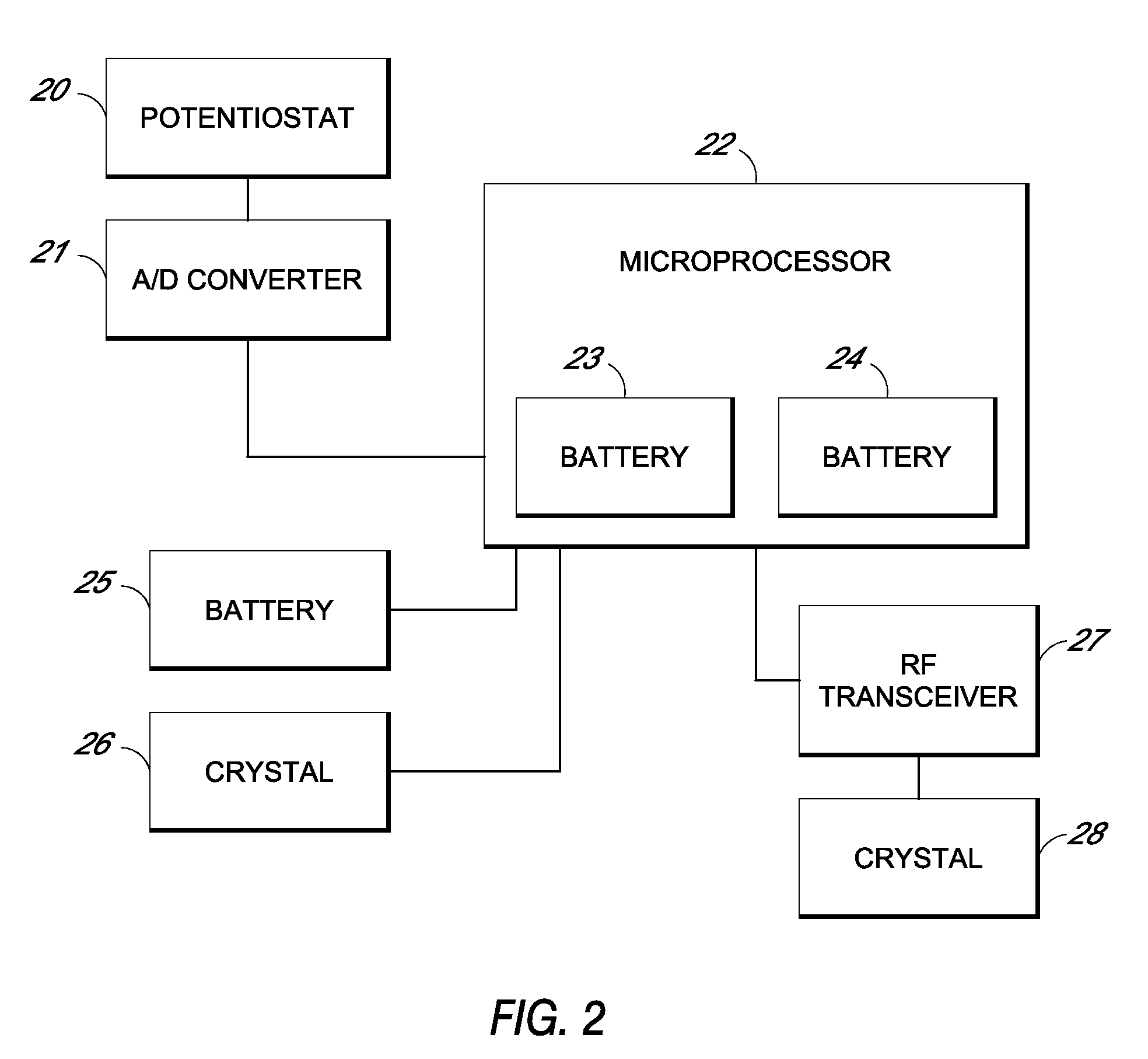
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
System and methods for processing analyte sensor data
ActiveUS20050027463A1Material thermal conductivityMaterial analysis by electric/magnetic meansAnalyteData system
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
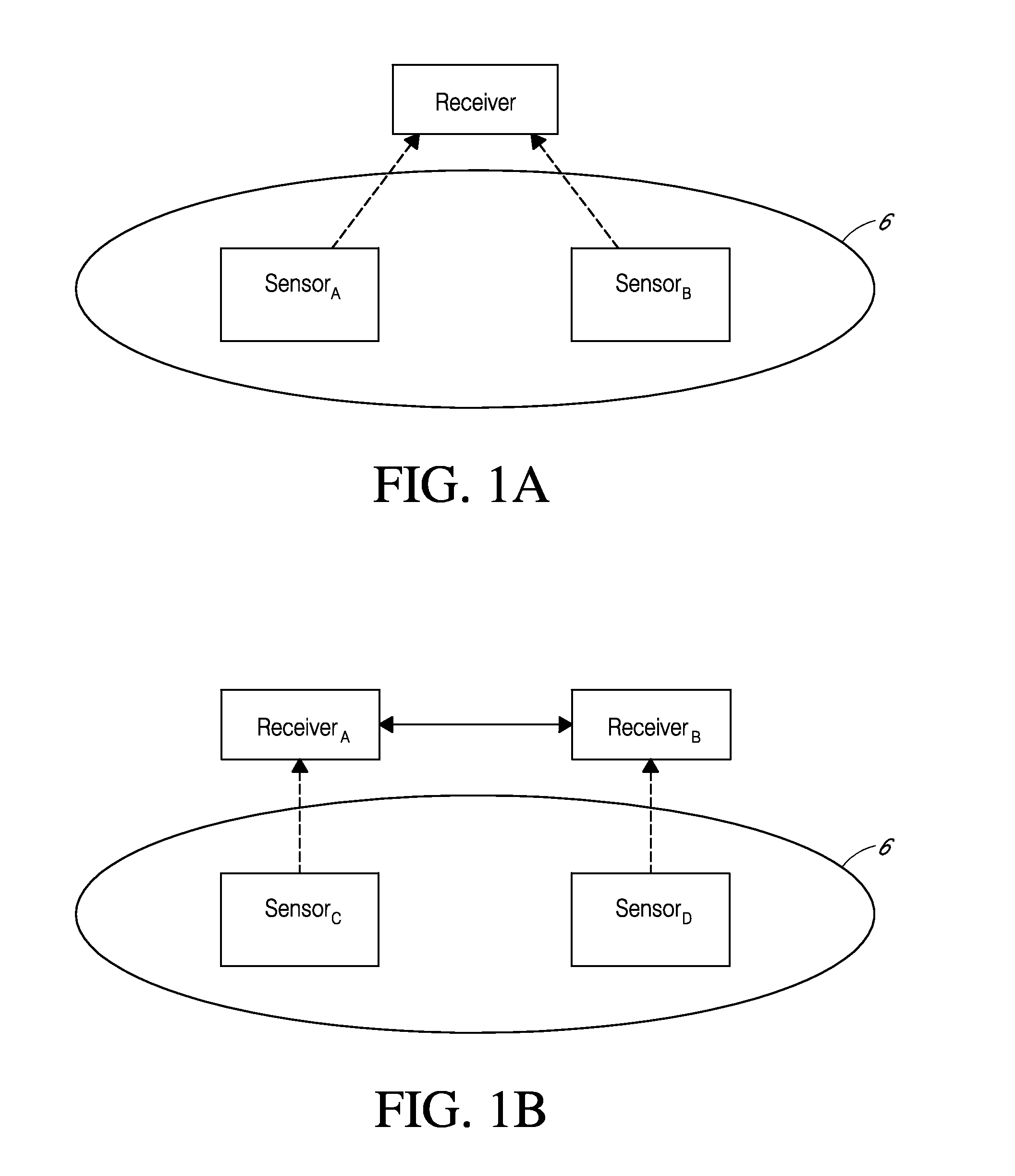
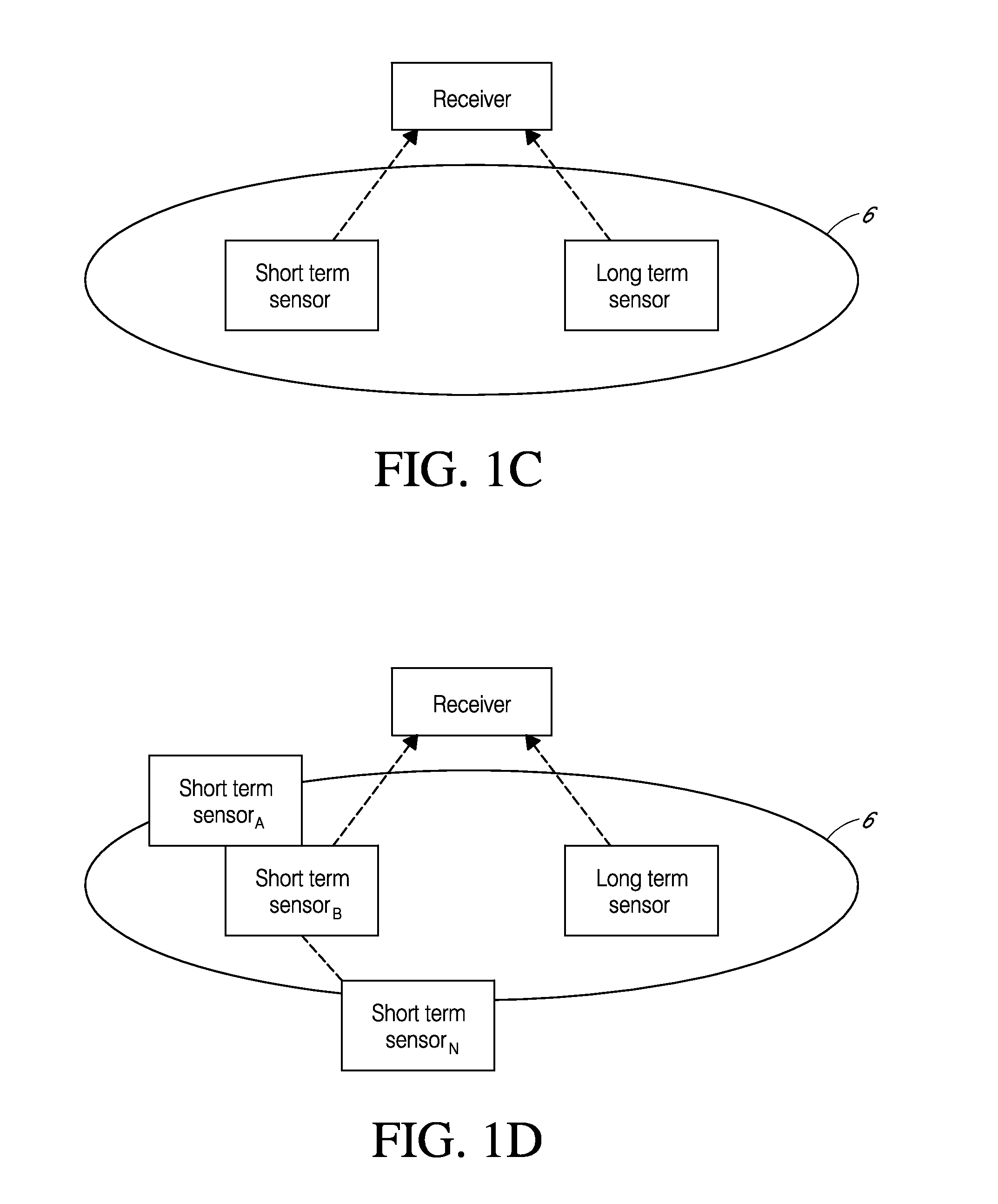
System and methods for processing analyte sensor data for sensor calibration
Systems and methods for processing sensor analyte data are disclosed, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. The sensor can be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. Reference data resulting from benchtop testing an analyte sensor prior to its insertion can be used to provide initial calibration of the sensor data. Reference data from a short term continuous analyte sensor implanted in a user can be used to initially calibrate or update sensor data from a long term continuous analyte sensor.
Owner:DEXCOM INC
System and methods for processing analyte sensor data
InactiveUS20110231140A1Testing/calibration apparatusSpeed measurement using gyroscopic effectsAnalyteData system
Owner:DEXCOM INC
System and methods for processing analyte sensor data
ActiveUS20110231142A1Testing/calibration apparatusSpeed measurement using gyroscopic effectsAnalyteData system
Owner:DEXCOM INC
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
System and methods for processing analyte sensor data for sensor calibration
Systems and methods for processing sensor analyte data are disclosed, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. The sensor can be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. Reference data resulting from benchtop testing an analyte sensor prior to its insertion can be used to provide initial calibration of the sensor data. Reference data from a short term continuous analyte sensor implanted in a user can be used to initially calibrate or update sensor data from a long term continuous analyte sensor.
Owner:DEXCOM INC
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
Method for optical measurements of tissue to determine disease state or concentration of an analyte
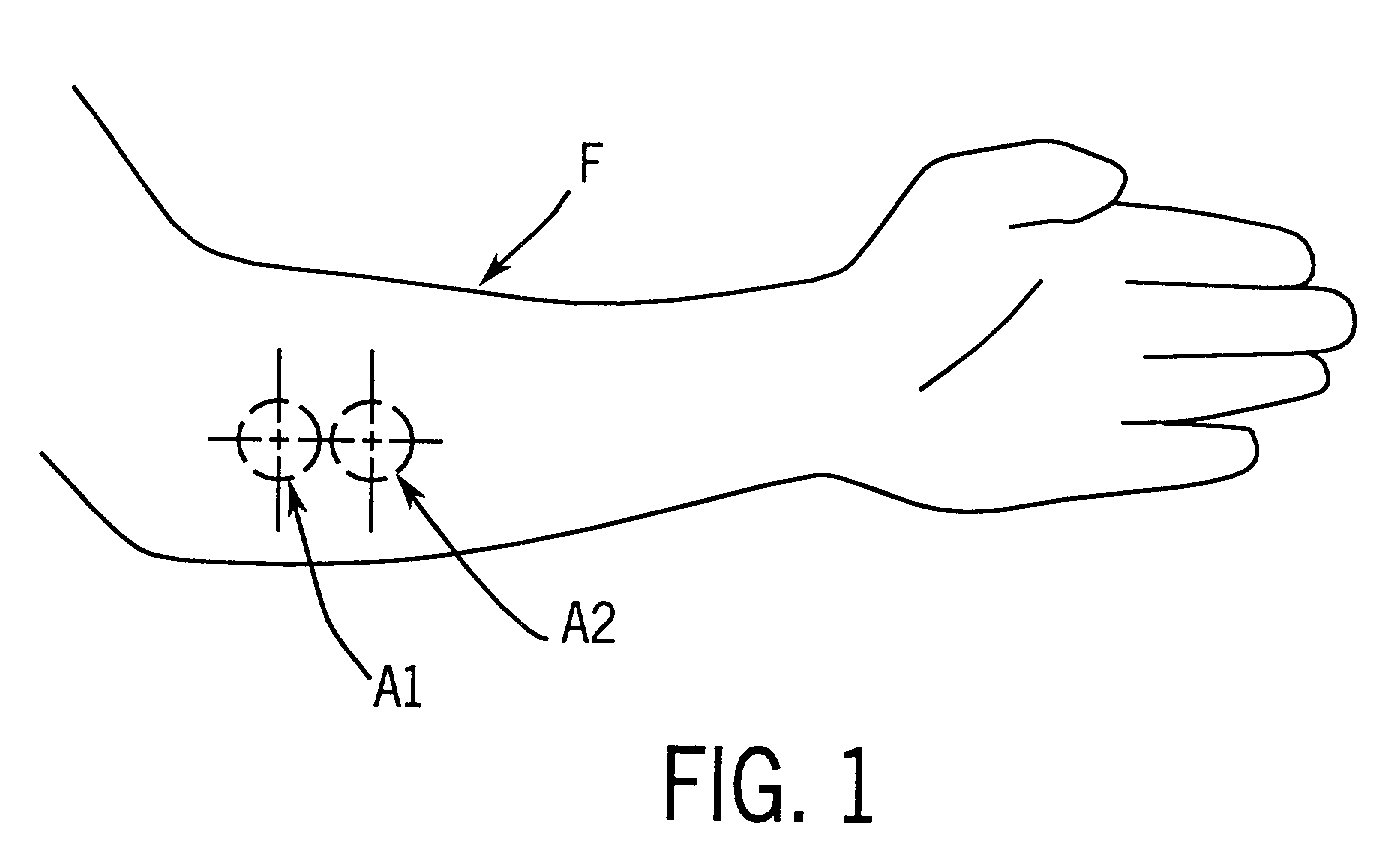
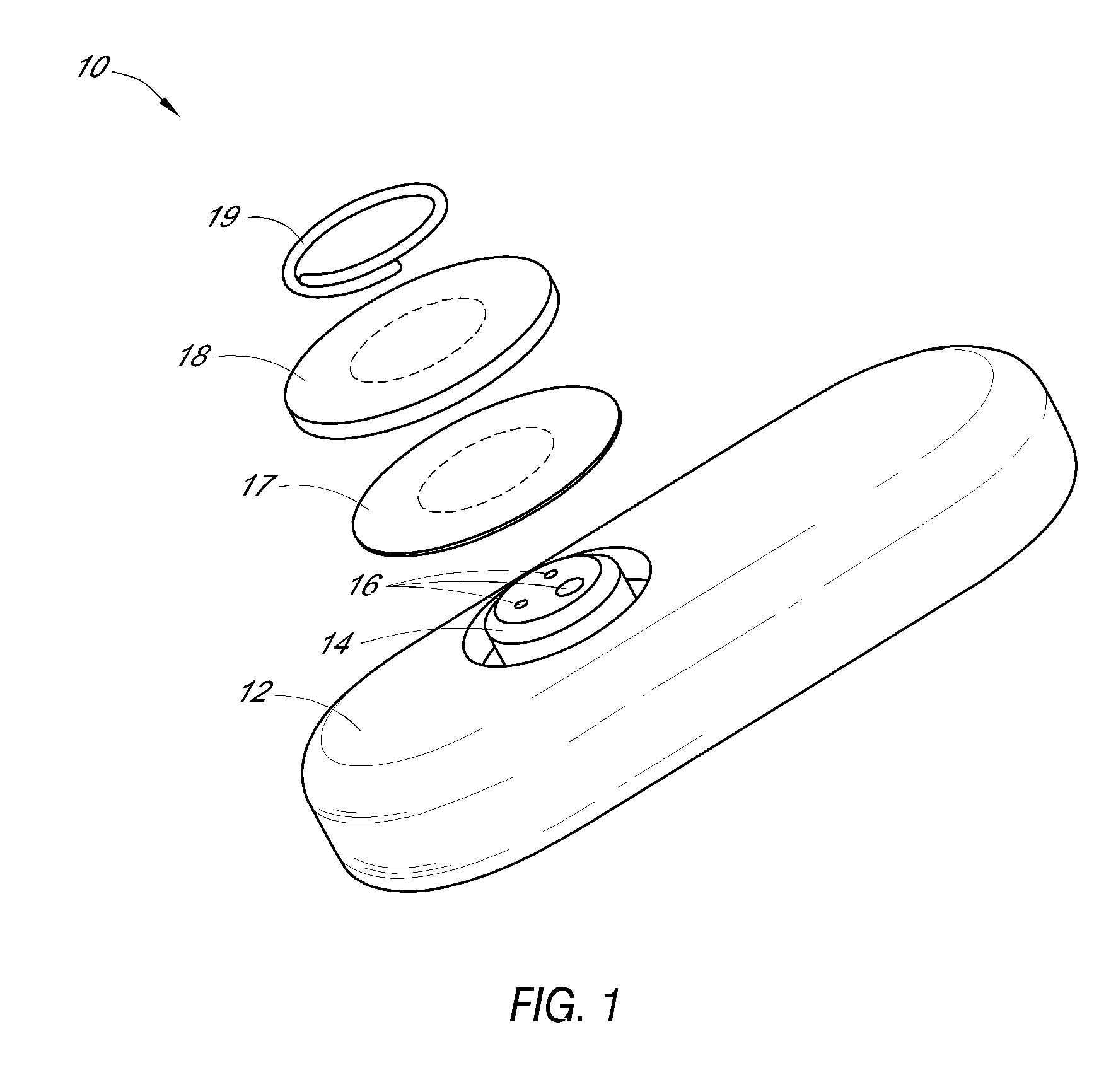
A method for collecting optical data at two morphologically similar, substantially non-overlapping, and preferably adjacent, areas on the surface of a tissue, while the temperature in each area is being maintained or modulated according to a temperature program. The optical data obtained are inserted into a mathematical relationship, e.g., an algorithm, that can be used to predict a disease state (such as the diabetes mellitus disease state) or the concentration of an analyte for indicating a physical condition (such as blood glucose level). This invention can be used to differentiate between disease status, such as, for example, diabetic and non-diabetic. The method involves the generation of a calibration (or training) set that utilizes the relationship between optical signals emanating from the skin under different thermal stimuli and disease status, e.g., diabetic status, established clinically. This calibration set can be used to predict the disease state of other subjects. Structural changes, as well as circulatory changes, due to a disease state are determined at two morphologically similar, but substantially non-overlapping areas on the surface of human tissue, e.g., the skin of a forearm, with each area being subjected to different temperature modulation programs. In addition to determination of a disease state, this invention can also be used to determine the concentration of an analyte in the tissues. This invention also provides an apparatus for the determination of a disease state, such as diabetes, or concentration of an analyte, such as blood glucose level, by the method of this invention.
Owner:ABBOTT DIABETES CARE INC
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM INC
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM INC
System and methods for processing analyte sensor data
Systems and methods for processing sensor analyte data, including initiating calibration, updating calibration, evaluating clinical acceptability of reference and sensor analyte data, and evaluating the quality of sensor calibration. During initial calibration, the analyte sensor data is evaluated over a period of time to determine stability of the sensor. The sensor may be calibrated using a calibration set of one or more matched sensor and reference analyte data pairs. The calibration may be updated after evaluating the calibration set for best calibration based on inclusion criteria with newly received reference analyte data. Fail-safe mechanisms are provided based on clinical acceptability of reference and analyte data and quality of sensor calibration. Algorithms provide for optimized prospective and retrospective analysis of estimated blood analyte data from an analyte sensor.
Owner:DEXCOM
Near infrared spectrum nondestructive testing method and device for material component content
InactiveCN101915744AReduce complexityImprove modeling efficiencyColor/spectral properties measurementsInfraredNonlinear calibration
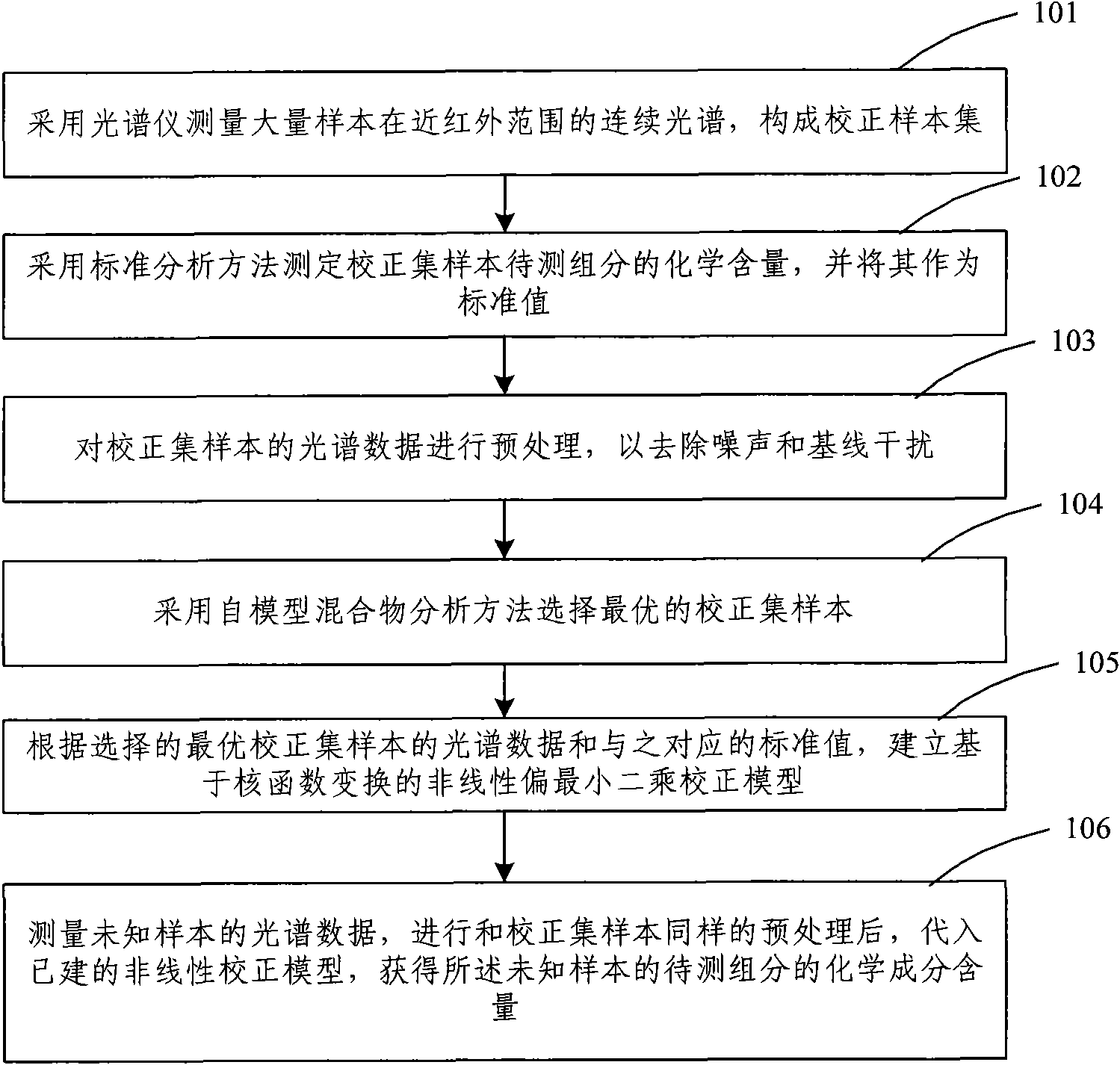
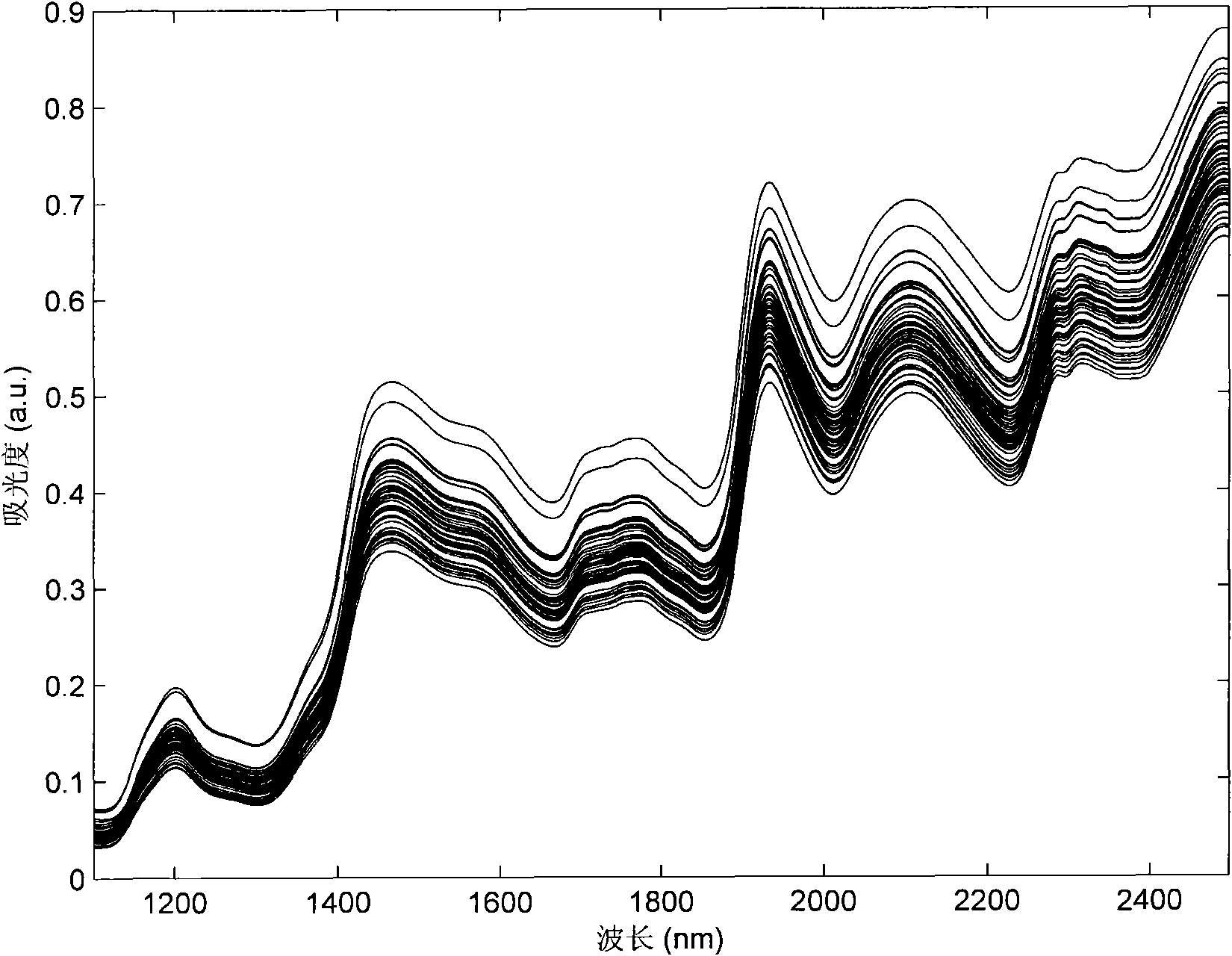
The invention discloses a near infrared spectrum nondestructive testing method and a near infrared spectrum nondestructive testing device for material component content. The method comprises the following steps of: collecting calibration set samples by using a spectrometer, pretreating a spectrum, selecting an optimal calibration sample set through sample optimization and establishing a nonlinear calibration model by using the optimal calibration sample set, collecting spectrum samples with unknown component content by using the spectrometer, pretreating the spectrum in a mode which is the same as that of pretreating the calibration set samples, and detecting the component content of the unknown sample through the established nonlinear calibration model. The method and the device can effectively solve the problems of complex calibration model, slow training speed and difficult hardware implementation in the conventional near infrared nondestructive testing method for the material component content and obviously improve the accuracy and stability of a nondestructive testing result of the material component content.
Owner:BEIHANG UNIV
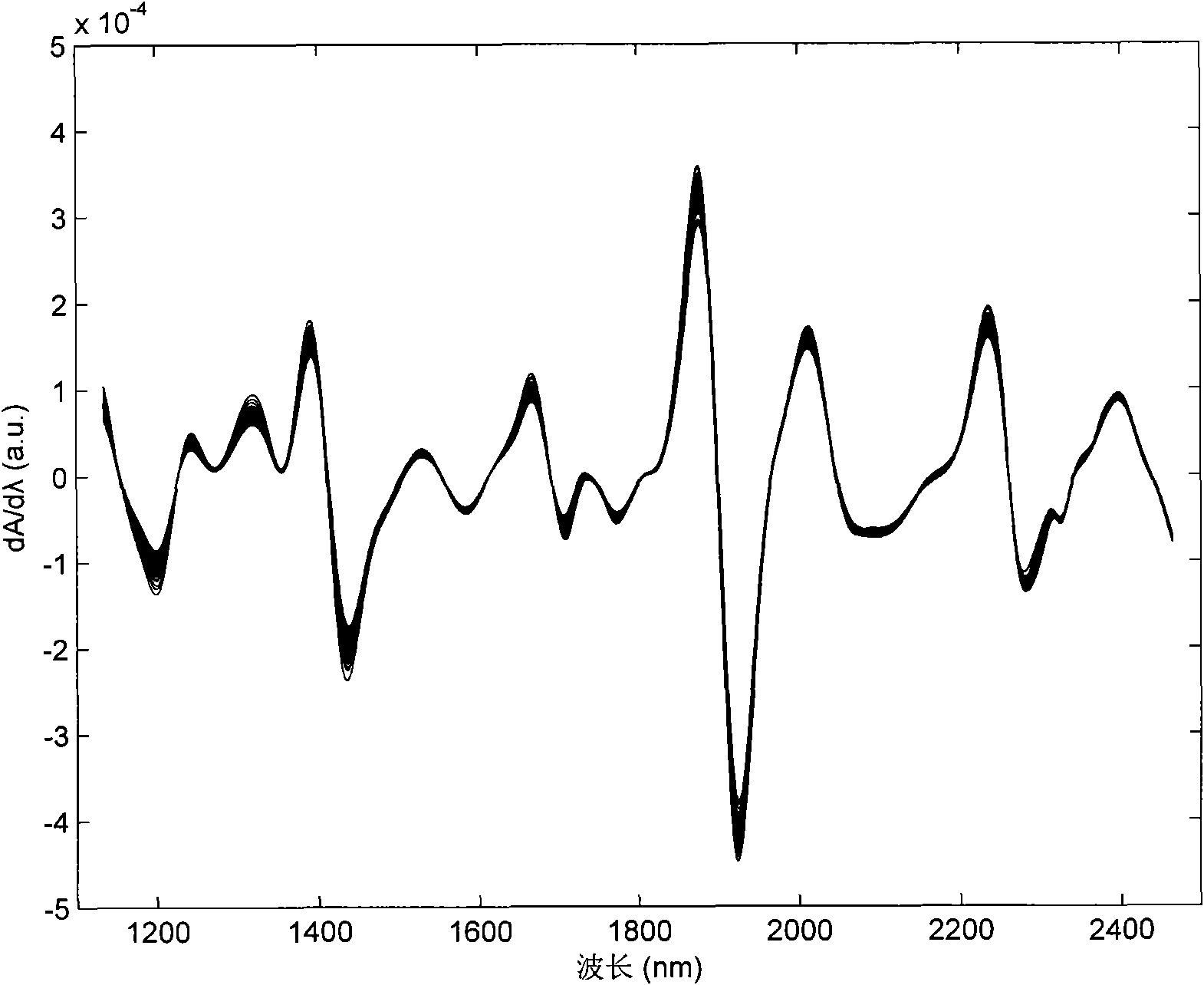
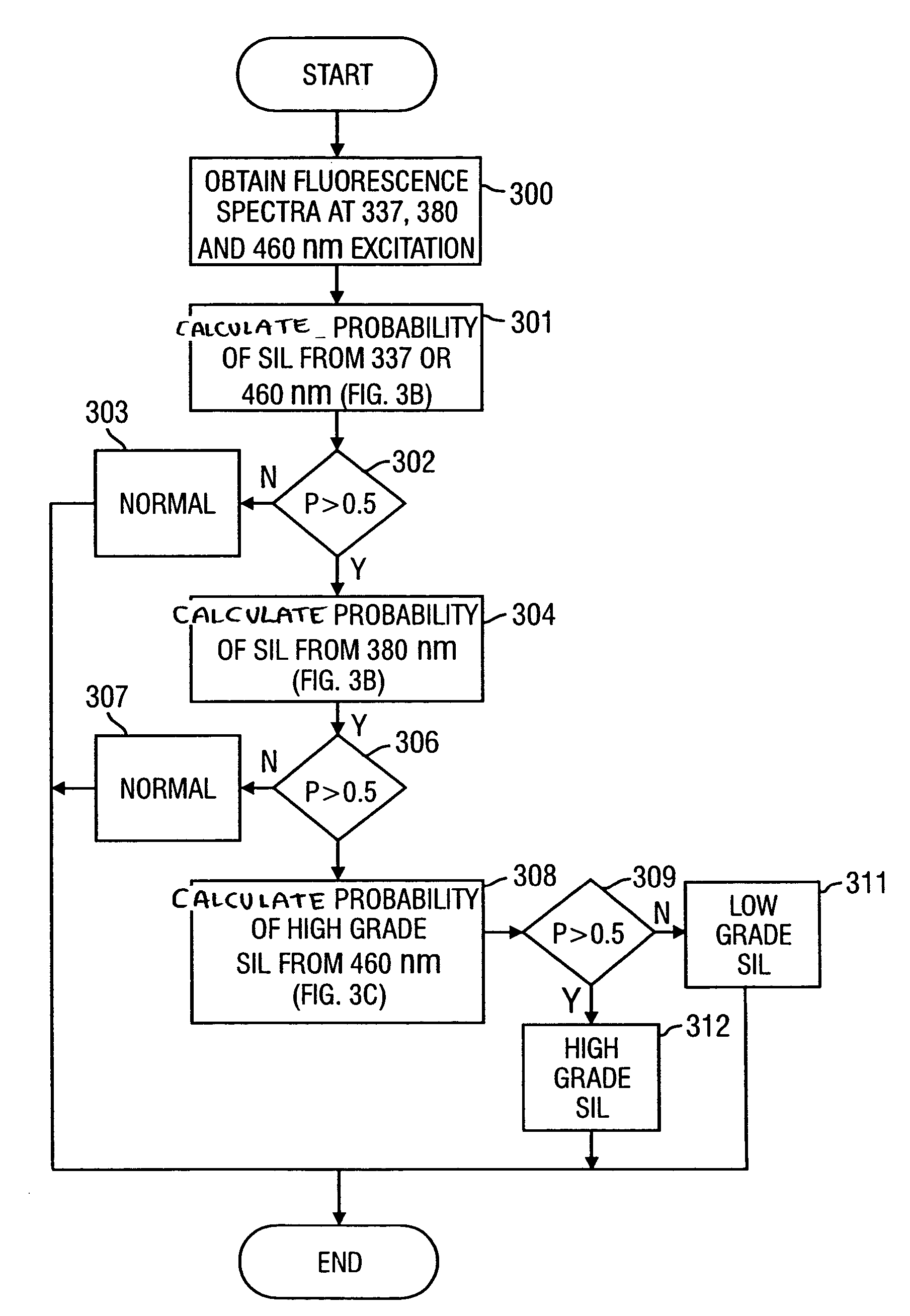
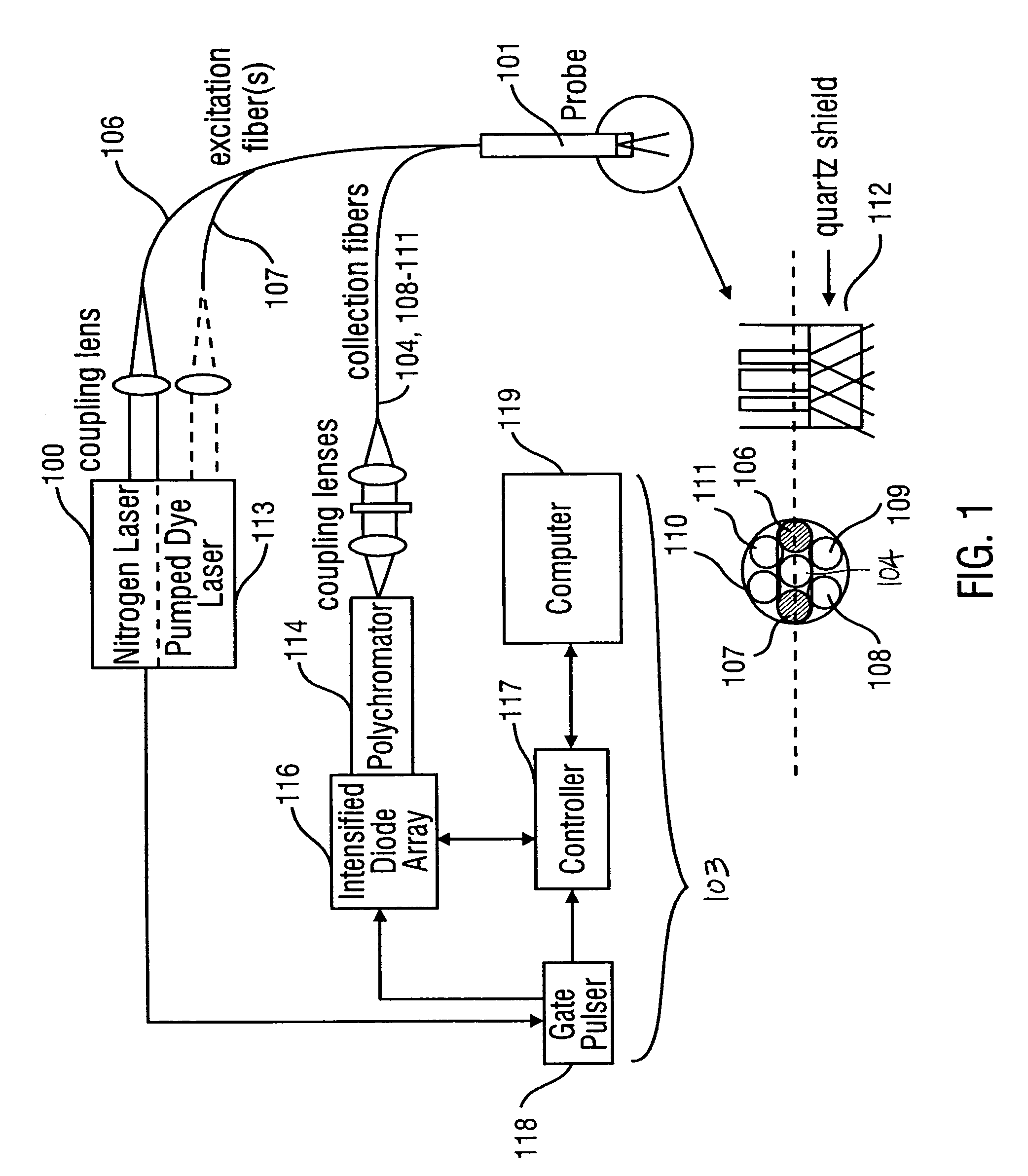
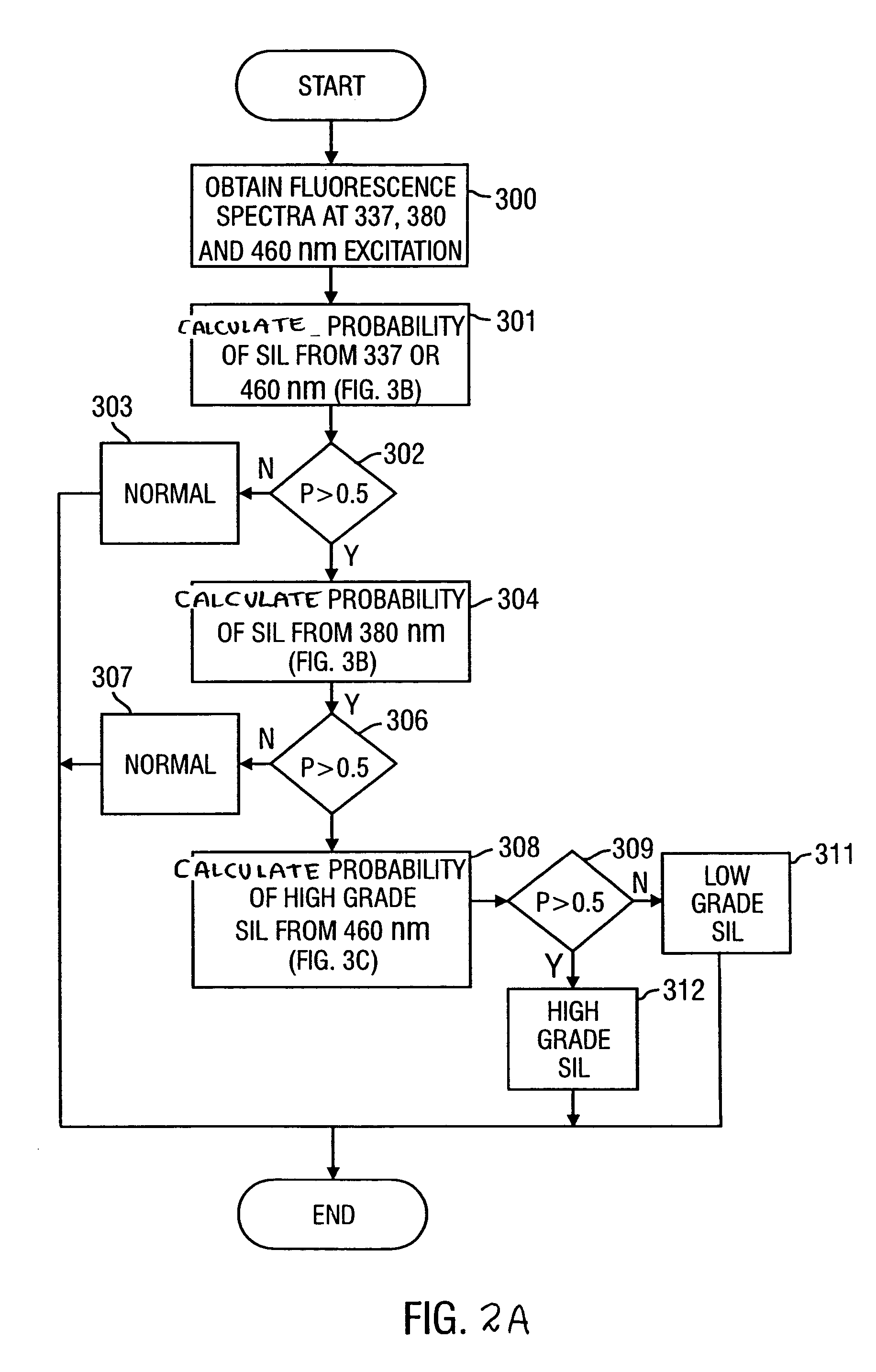
Method for probabilistically classifying tissue in vitro and in vivo using fluorescence spectroscopy
InactiveUS7236815B2Faster and effective managementReduce mortalityDiagnostics using spectroscopyDiagnostics using fluorescence emissionMultivariate statisticalPrincipal component analysis
Fluorescence spectral data acquired from tissues in vivo or in vitro is processed in accordance with a multivariate statistical method to achieve the ability to probabilistically classify tissue in a diagnostically useful manner, such as by histopathological classification. The apparatus includes a controllable illumination device for emitting electromagnetic radiation selected to cause tissue to produce a fluorescence intensity spectrum. Also included are an optical system for applying the plurality of radiation wavelengths to a tissue sample, and a fluorescence intensity spectrum detecting device for detecting an intensity of fluorescence spectra emitted by the sample as a result of illumination by the controllable illumination device. The system also include a data processor, connected to the detecting device, for analyzing detected fluorescence spectra to calculate a probability that the sample belongs in a particular classification. The data processor analyzes the detected fluorescence spectra using a multivariate statistical method. The five primary steps involved in the multivariate statistical method are (i) preprocessing of spectral data from each patient to account for inter-patient variation, (ii) partitioning of the preprocessed spectral data from all patients into calibration and prediction sets, (iii) dimension reduction of the preprocessed spectra in the calibration set using principal component analysis, (iv) selection of the diagnostically most useful principal components using a two-sided unpaired student's t-test and (v) development of an optimal classification scheme based on logistic discrimination using the diagnostically useful principal component scores of the calibration set as inputs.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
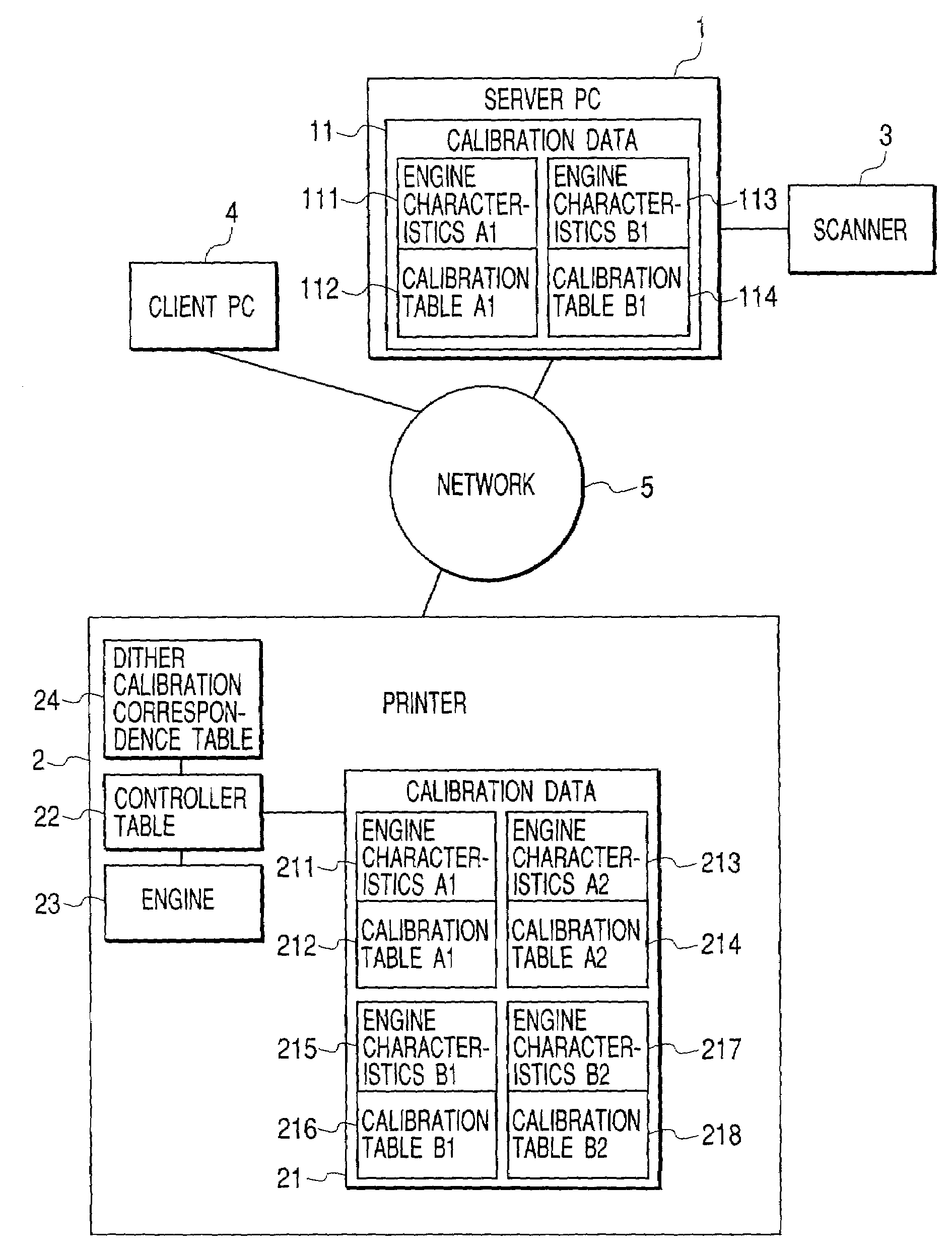
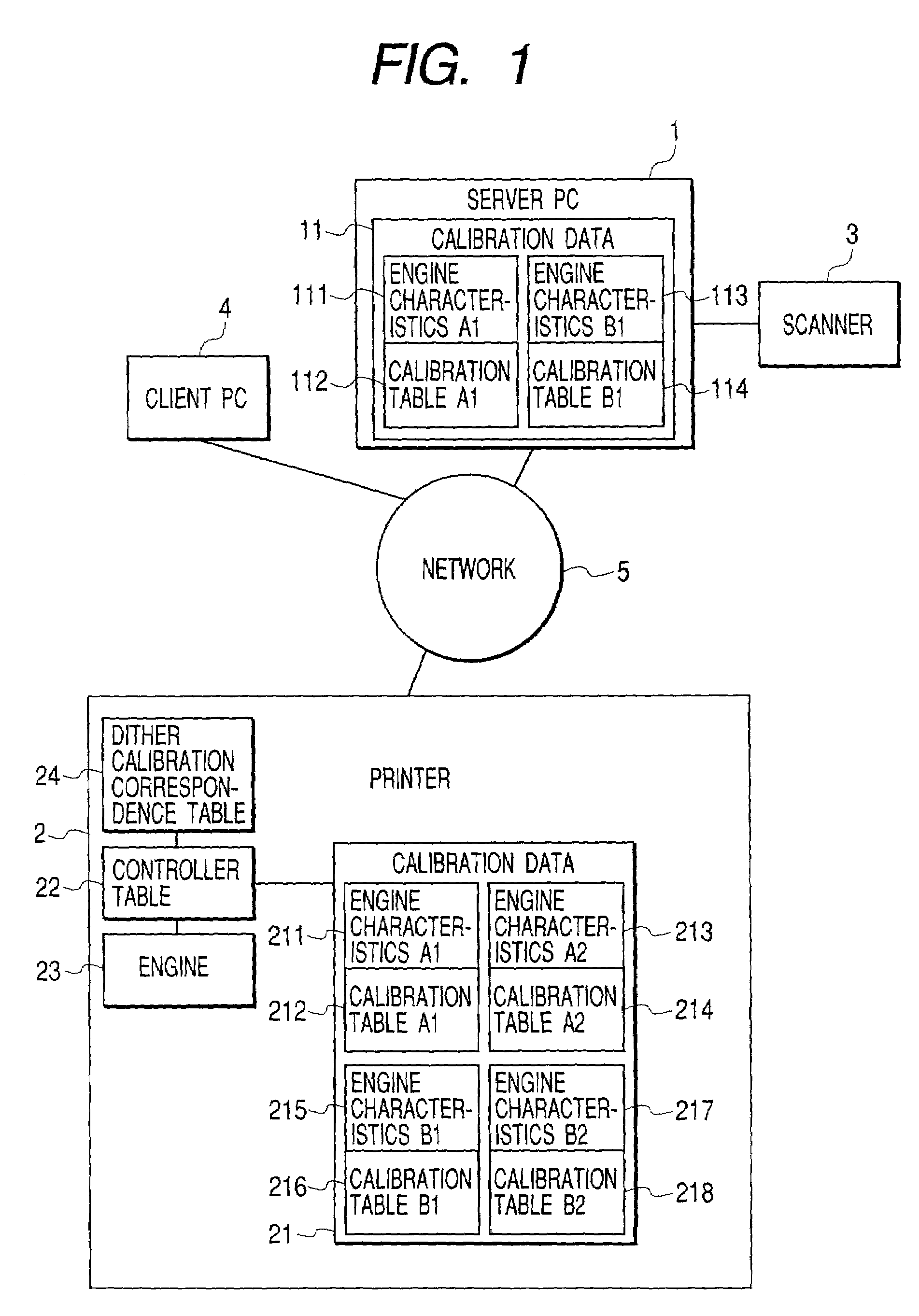
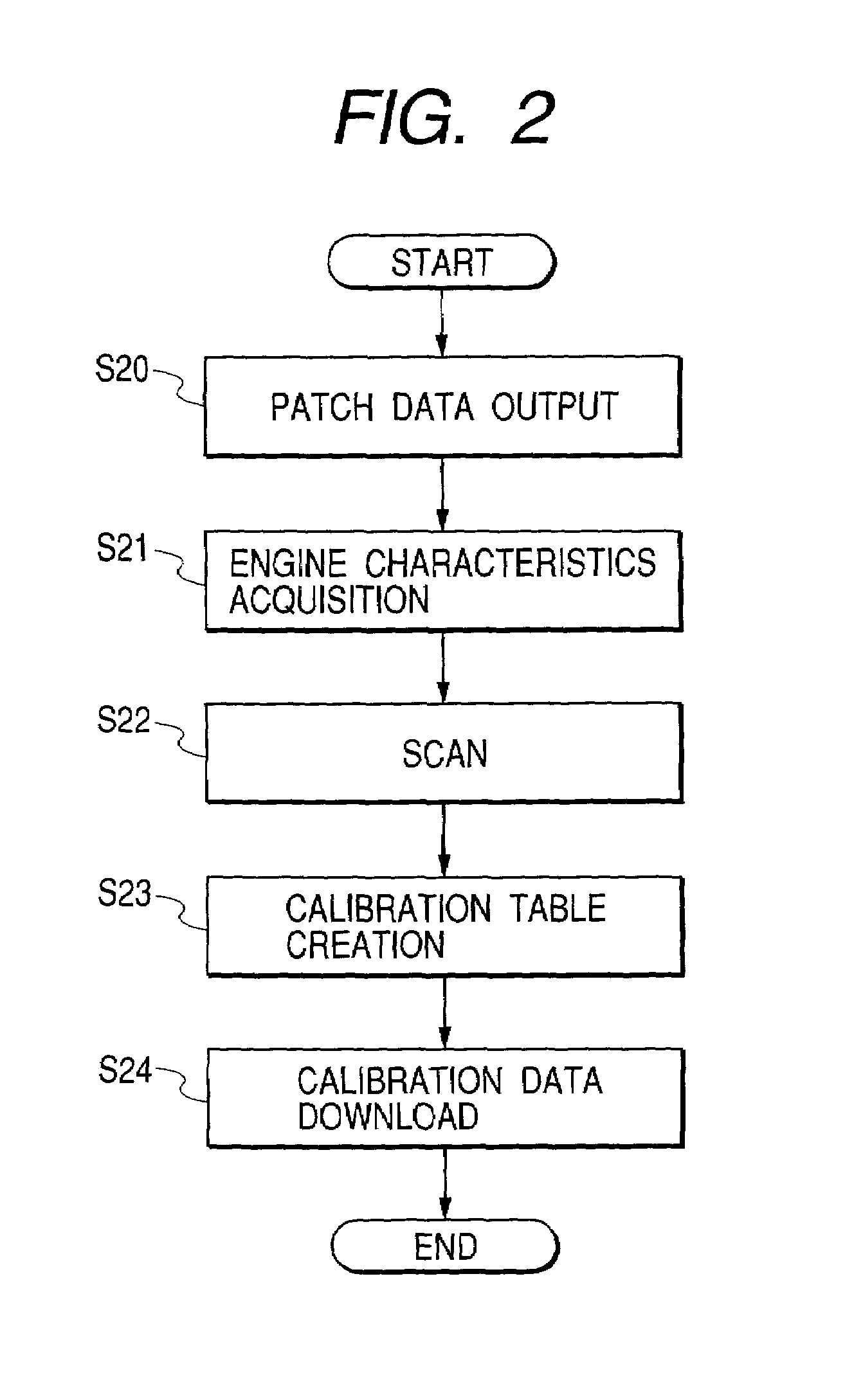
Calibration method for density in image forming apparatus
InactiveUS7061648B2Accurate CalibrationImage enhancementDigitally marking record carriersImage formationVolumetric Mass Density
Calibration associated with output density correction of a printer is effected by software calibration manipulated by the user and device calibration automatically performed by the printer, and, regarding these calibrations, high accurate calibration in which dither patterns for binarizing processing are matched to each other is effected.In a system in which either one of halftone patterns A, B, C and D as dither patterns can be used, regarding fewer number of patterns A and B, second calibration tables are created by correcting first calibration tables based on the software calibration by using correction data of engine characteristics based on the device calibration. Among the usable halftone patterns, the calibration table corresponding to the pattern A or B is selected in accordance with the set halftone pattern, and the output density correction by using the selected table.
Owner:CANON KK
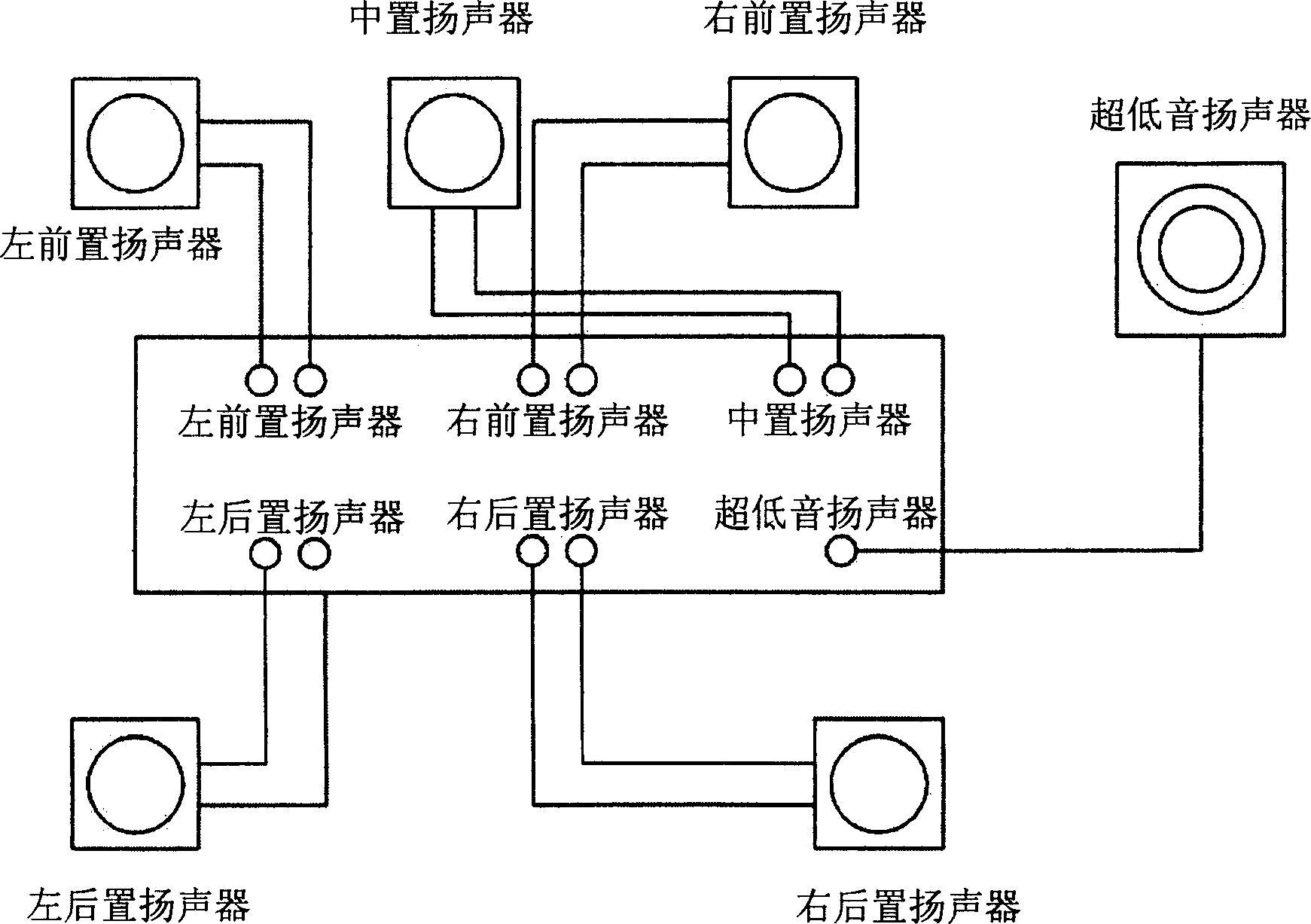
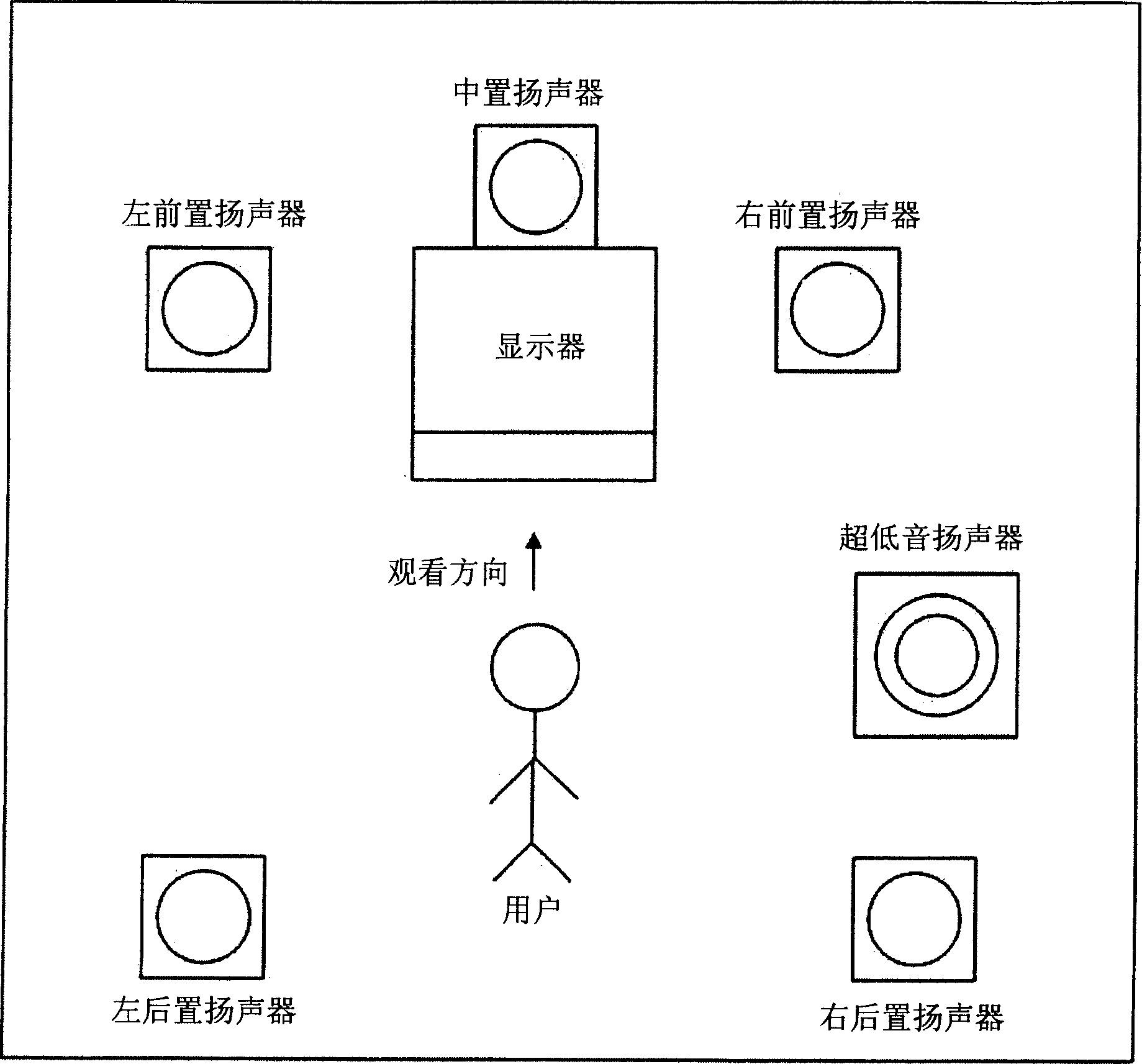
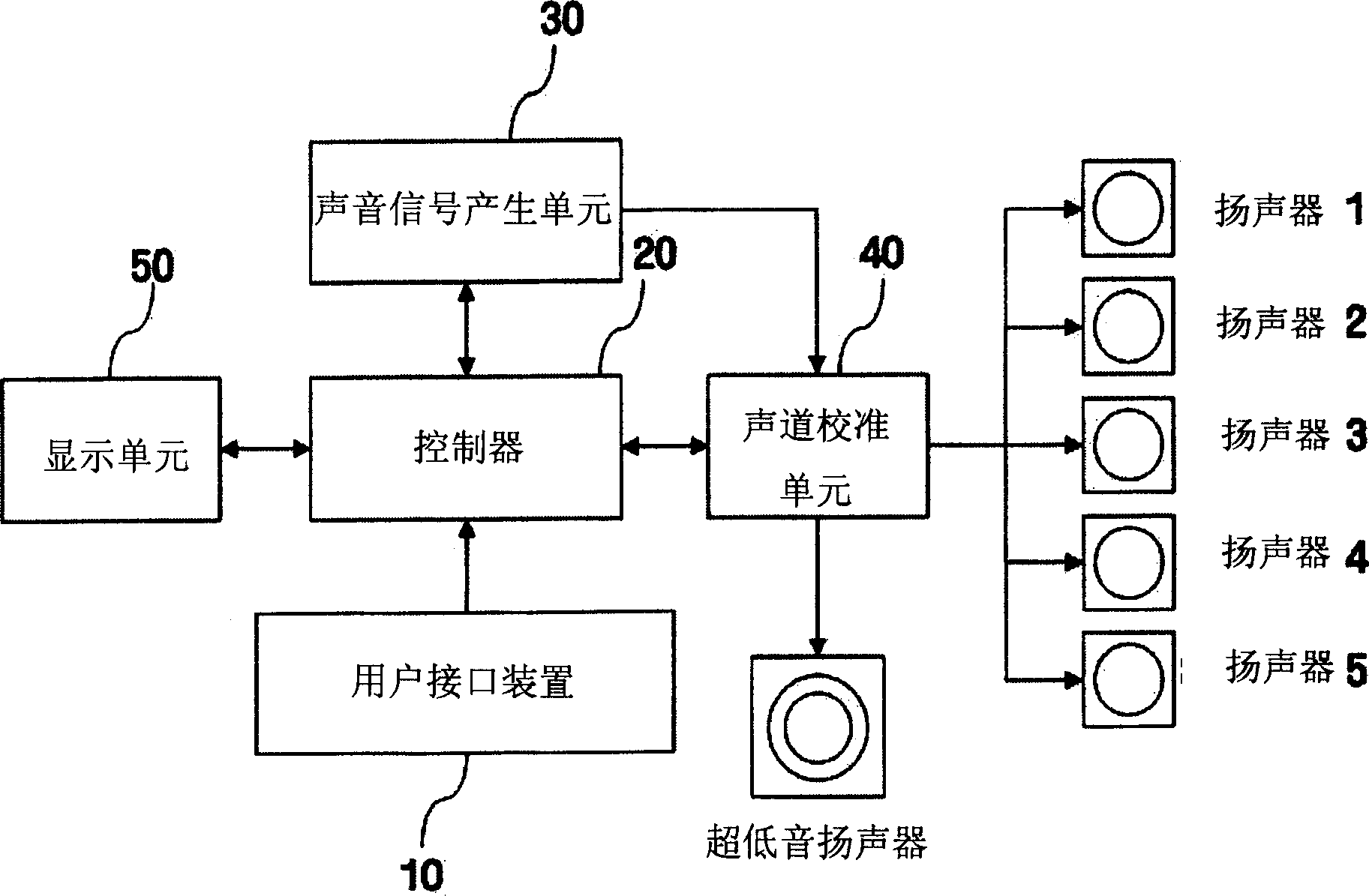
Method and equipment for playing multichannel digital sound
The invention discloses a method and equipment for multi-channel digital sound playback. The user can enjoy multi-channel music calibrated to properly match the user's location. The apparatus includes a sound signal generation unit for generating a digital sound signal from multi-channel sound resource data, and a channel calibration unit for storing a channel calibration matrix set by a control signal generated based on user's position information, using the The stored matrix calibrates the multi-channel sound signal and carries the calibrated sound signal to a plurality of speakers. A multi-channel digital sound playback method includes generating a multi-channel sound signal from multi-channel sound resource data, and setting a channel calibration matrix based on user's position information input by a user and calibrating the multi-channel sound signal using the set matrix Then carry the calibrated multi-channel sound signal to multiple speakers.
Owner:SAMSUNG ELECTRONICS CO LTD
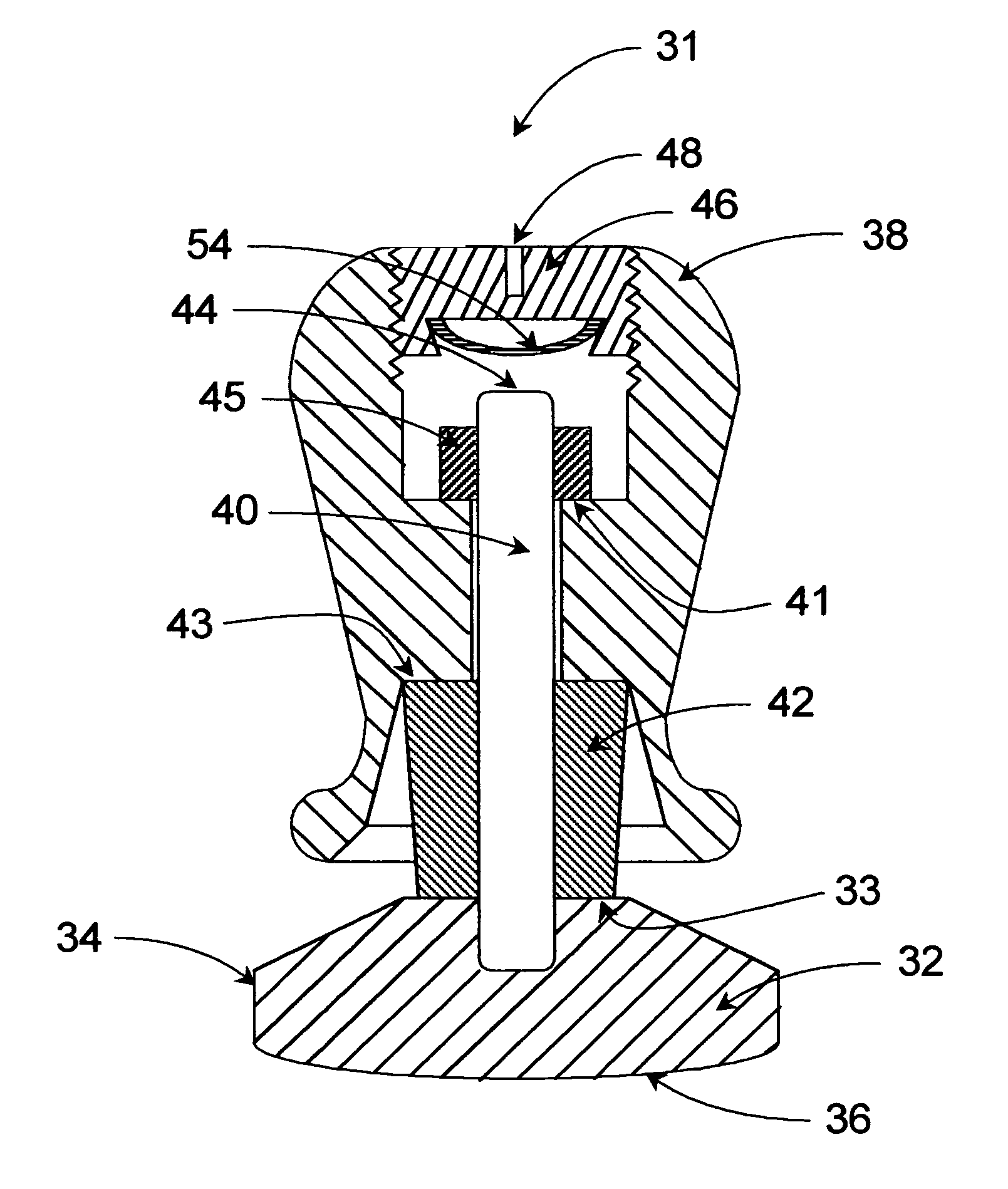
Calibrated handheld espresso tamper
An espresso tamper including a force calibration means is shown. The tamper is optimally handheld. Upon force of a desired magnitude being applied by a user, at least one discrete signal, such as an audible, visual, tactile or electrical signal is emitted. The tamper handle includes an anti-rotation feature. The handle is flared to avoid pinching the user's fingers during operation and to stop the user's fingers from sliding down the handle or in any way touching the base, thereby rendering the tamper more effective and safe. The tamper and force-calibration kit and method of use optimize espresso making.
Owner:ESPRO
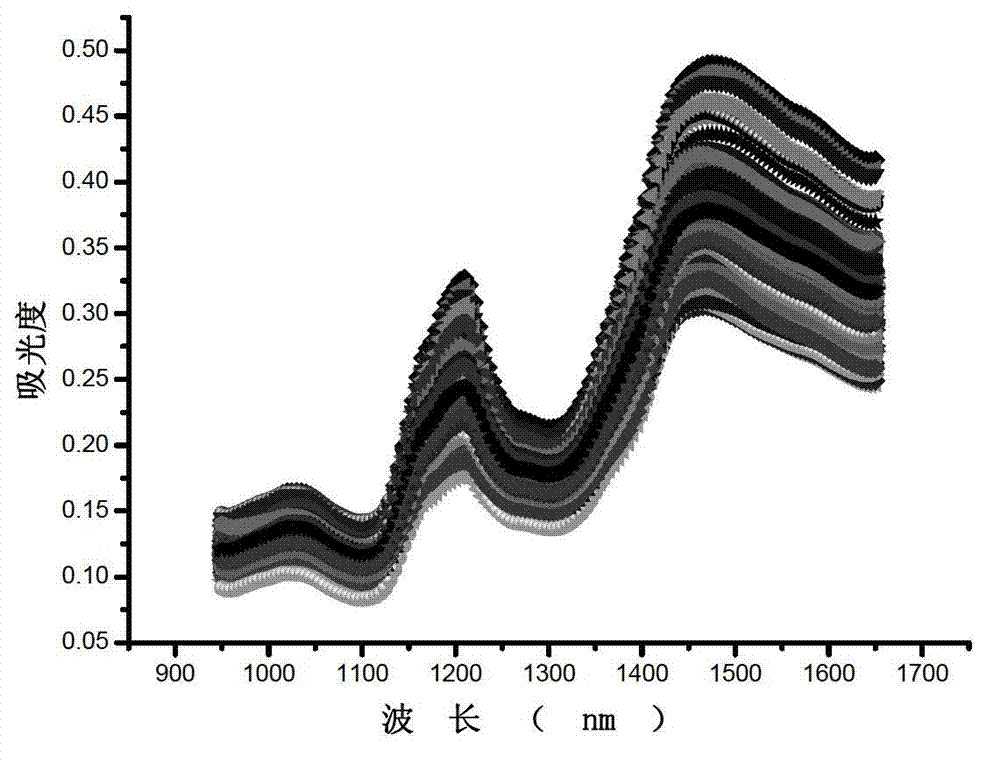
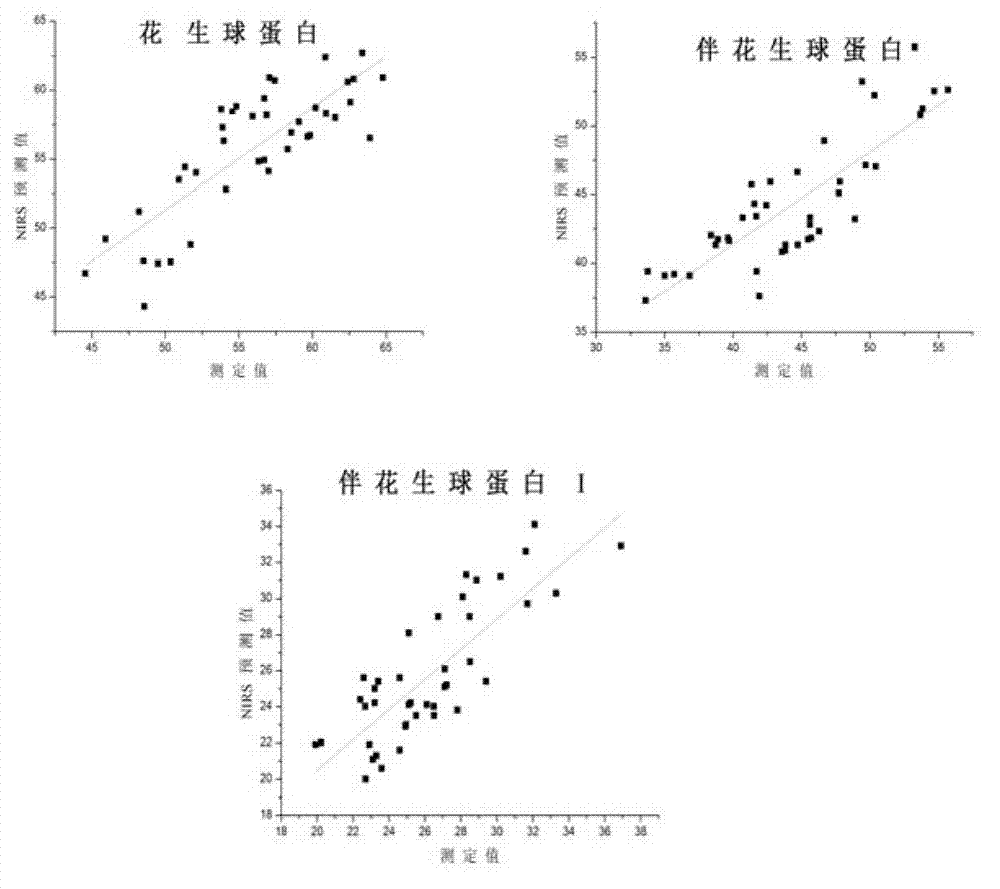
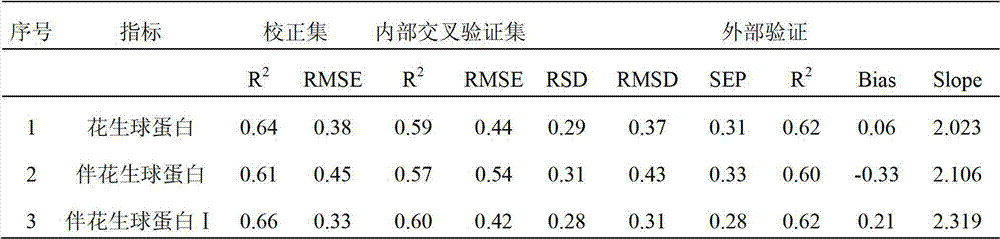
Near infrared detection method for contents of protein components in peanut
ActiveCN102879353ANo pollutionFast analysisColor/spectral properties measurementsQuality controlComputer science
The invention discloses a near infrared detection method for the contents of the protein components in a peanut, which comprises the steps of: 1) building a calibration set sample spectrum; 2) preprocessing the calibration set sample spectrum; 3) extracting the characteristic information data of the calibration set sample spectrum; 4) building a correcting model; and 5) analyzing a sample to be detected. A near infrared spectrum of the sample to be detected is input into the correcting model after the near infrared spectrum of the sample to be detected is preprocessed and the characteristic information is extracted, and the contents of protein components in a sample nut can be obtained. The near infrared detection method for the contents of the protein components in the peanut provided by the invention has the advantages of being fast in analysis speed, high in analysis efficiency, low in analysis cost, and avoiding the environment pollution caused by any chemical reagent and any pollution etc., so that a reliable basis is provided for peanut quality analysis, peanut quality control and product quality.
Owner:INST OF AGRO FOOD SCI & TECH CHINESE ACADEMY OF AGRI SCI
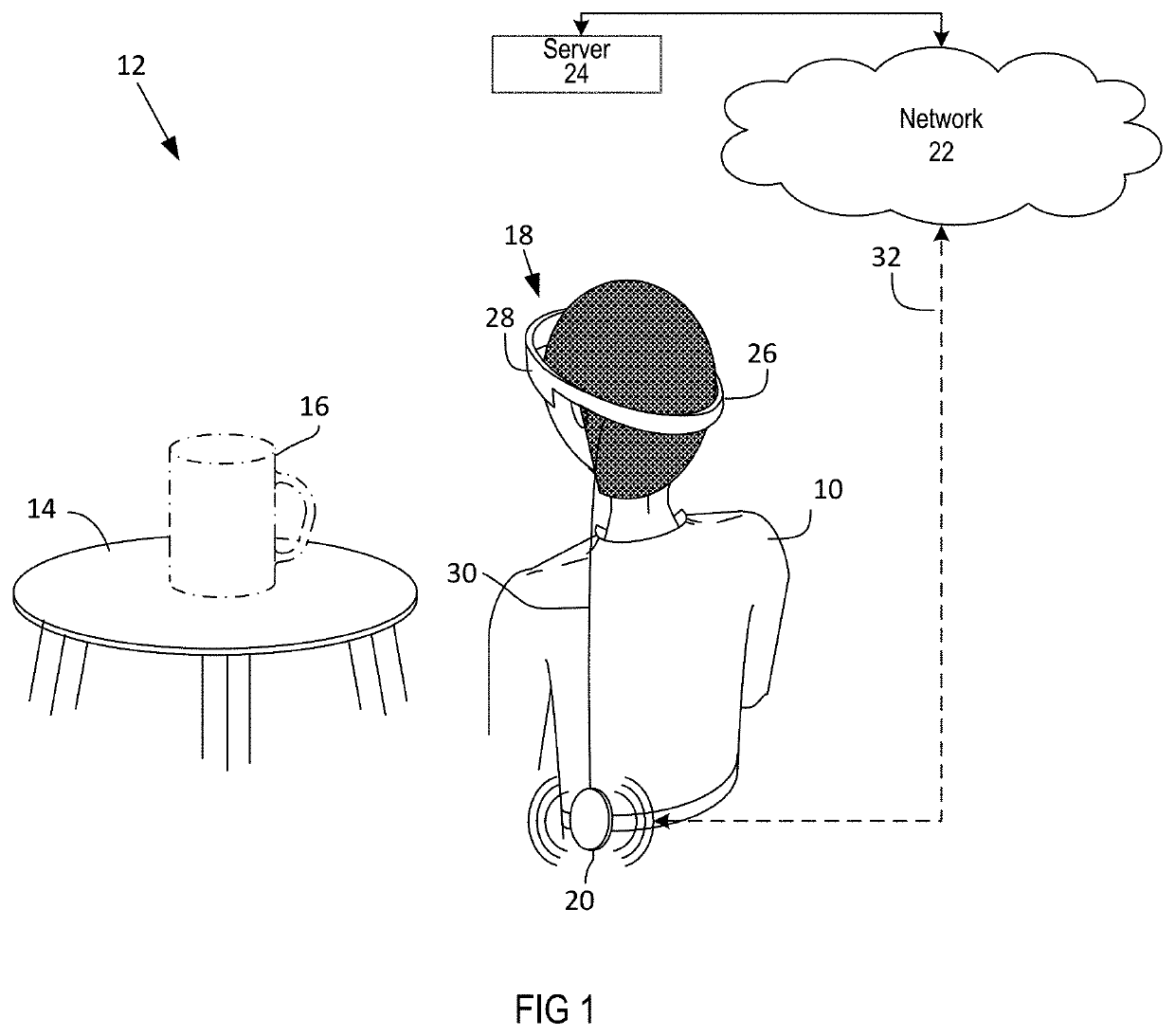
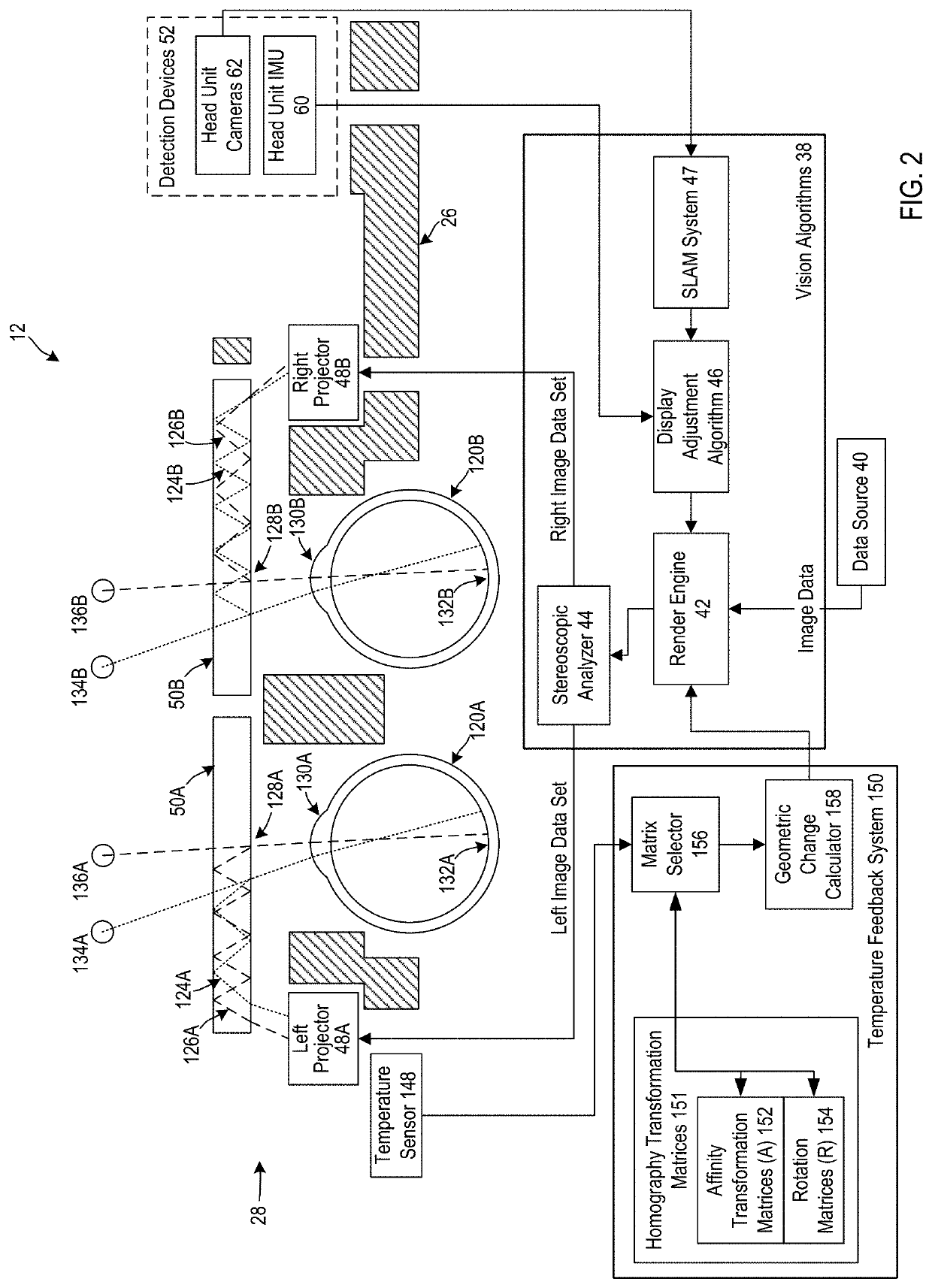
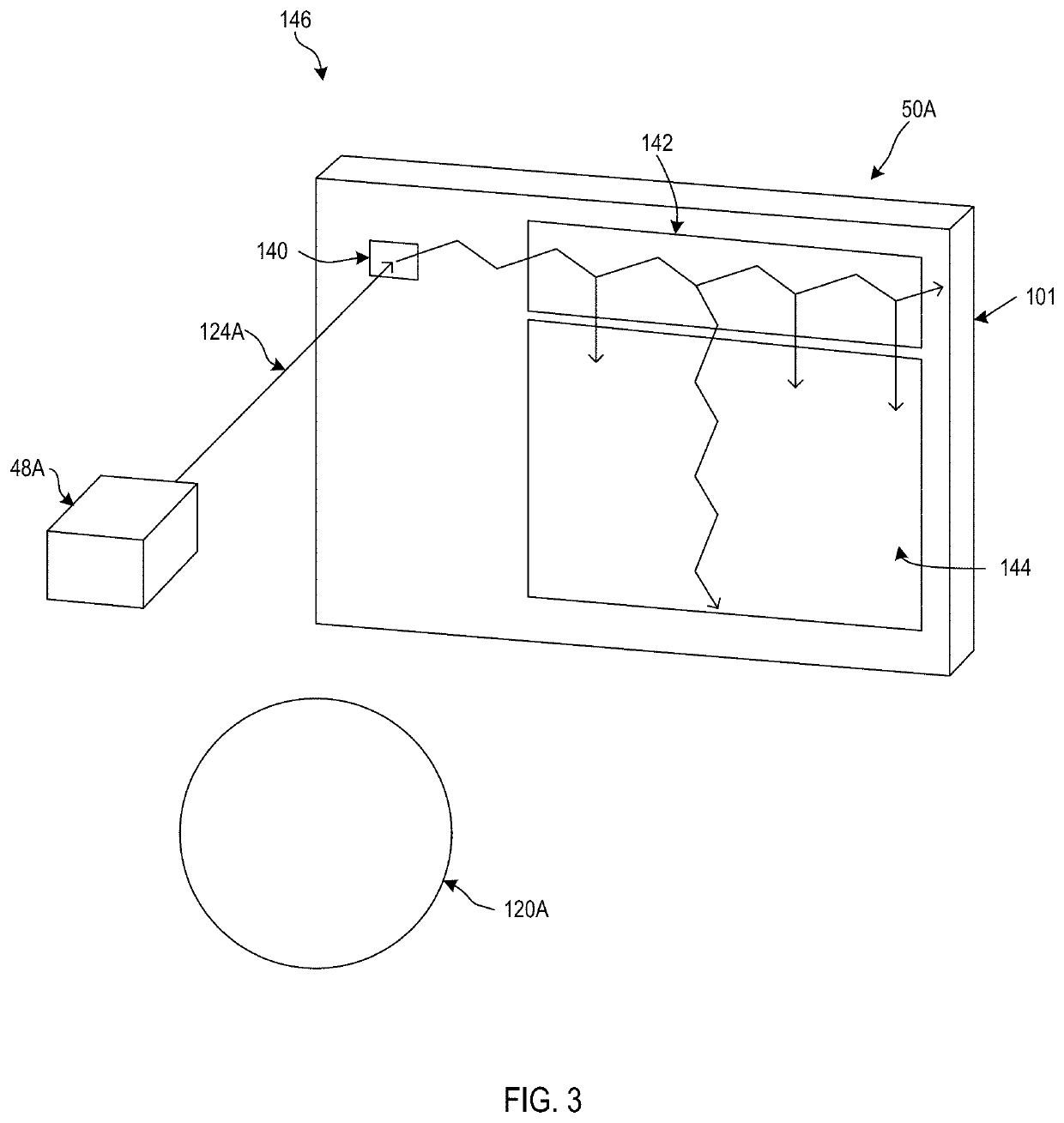
Homography transformation matrices based temperature calibration of a viewing system
Owner:MAGIC LEAP INC
Calibrated handheld espresso tamper
ActiveUS20050132890A1Application of tamping forceIncreasing tamping forceBeverage vesselsLiquid dispensingEngineeringCalibration set
An espresso tamper including a force calibration means is disclosed. The tamper is optimally handheld. Upon force of a desired magnitude being applied by a user, at least one discrete signal, such as an audible, visual, tactile or electrical signal is emitted. The tamper handle includes an anti-rotation feature. The handle is flared to avoid pinching the user's fingers during operation and to stop the user's fingers from sliding down the handle or in any way touching the base, thereby rendering the tamper more effective and safe. The tamper and force-calibration kit and method of use optimize espresso making.
Owner:ESPRO
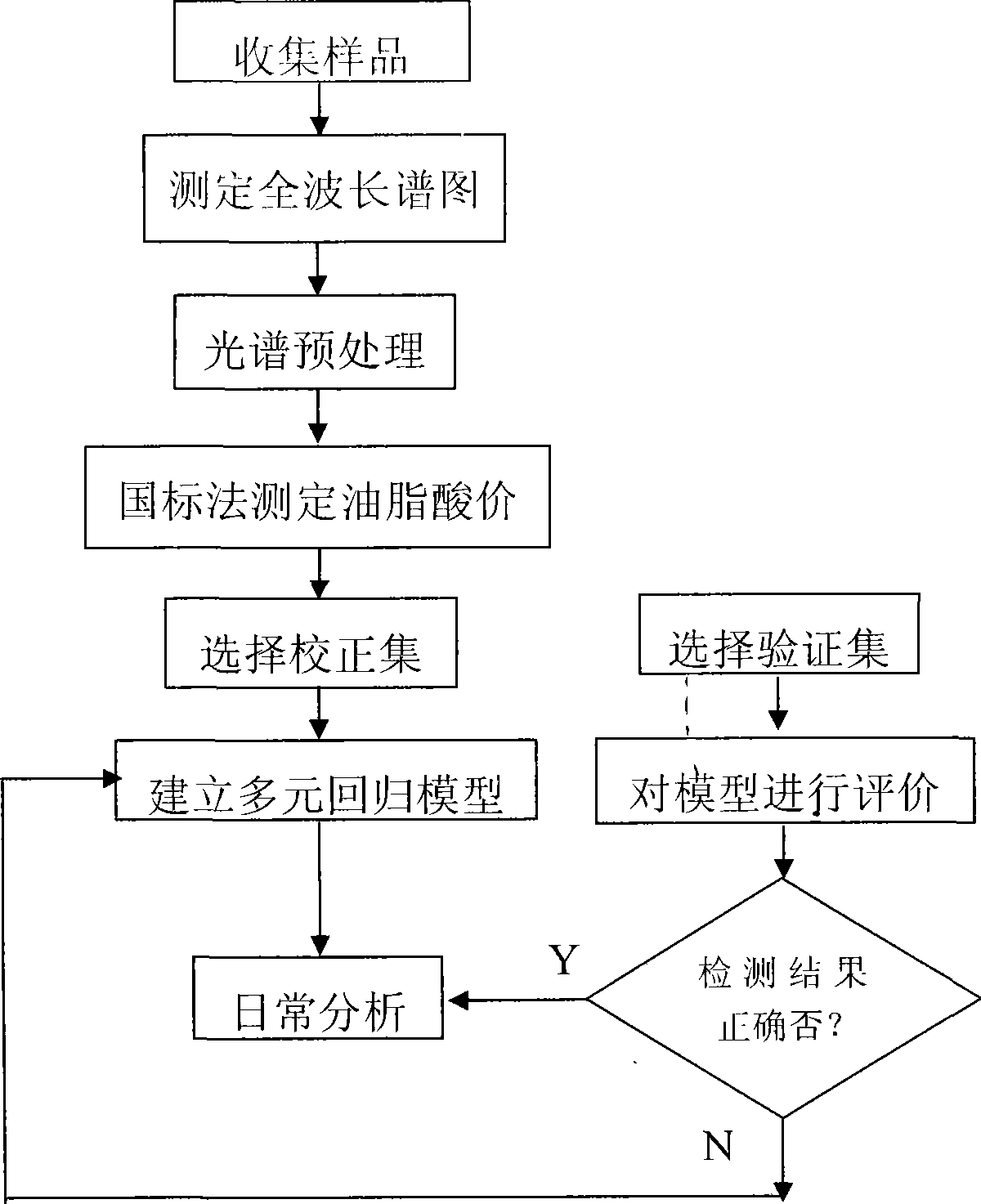
Edible fatty acid value detection method based on near-infrared spectrum analysis
InactiveCN101504363ARapid online real-time detection and analysisAccurate online real-time detection and analysisColor/spectral properties measurementsInfraredAcid value
The invention discloses a method for detecting an acid value of edible fat based on near infrared spectrum analysis. The invention relates to a method for detecting an acid value of edible fat by utilizing near infrared spectrum analysis technology, which aims to solve the problems that in the practical production, the prior laboratory detection and test method can only perform intermittent operation, fail to realize accurate and quick on-line detection and the like. The method for detecting the acid value of the edible fat by utilizing the near infrared spectrum analysis technology is realized through the following steps of: 1, establishment of a calibration set sample spectrum; 2, pretreatment of spectrum data; 3, determination of essential data; 4, establishment of a calibration model; 5, verification of the calibration model; and 6, analysis of a sample to be tested. The method can effectively eliminate personal error, shorten detection period, and realize the on-line detection and control of the acid value in the process of processing the fat.
Owner:HARBIN UNIV OF COMMERCE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com