System and methods for processing analyte sensor data
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
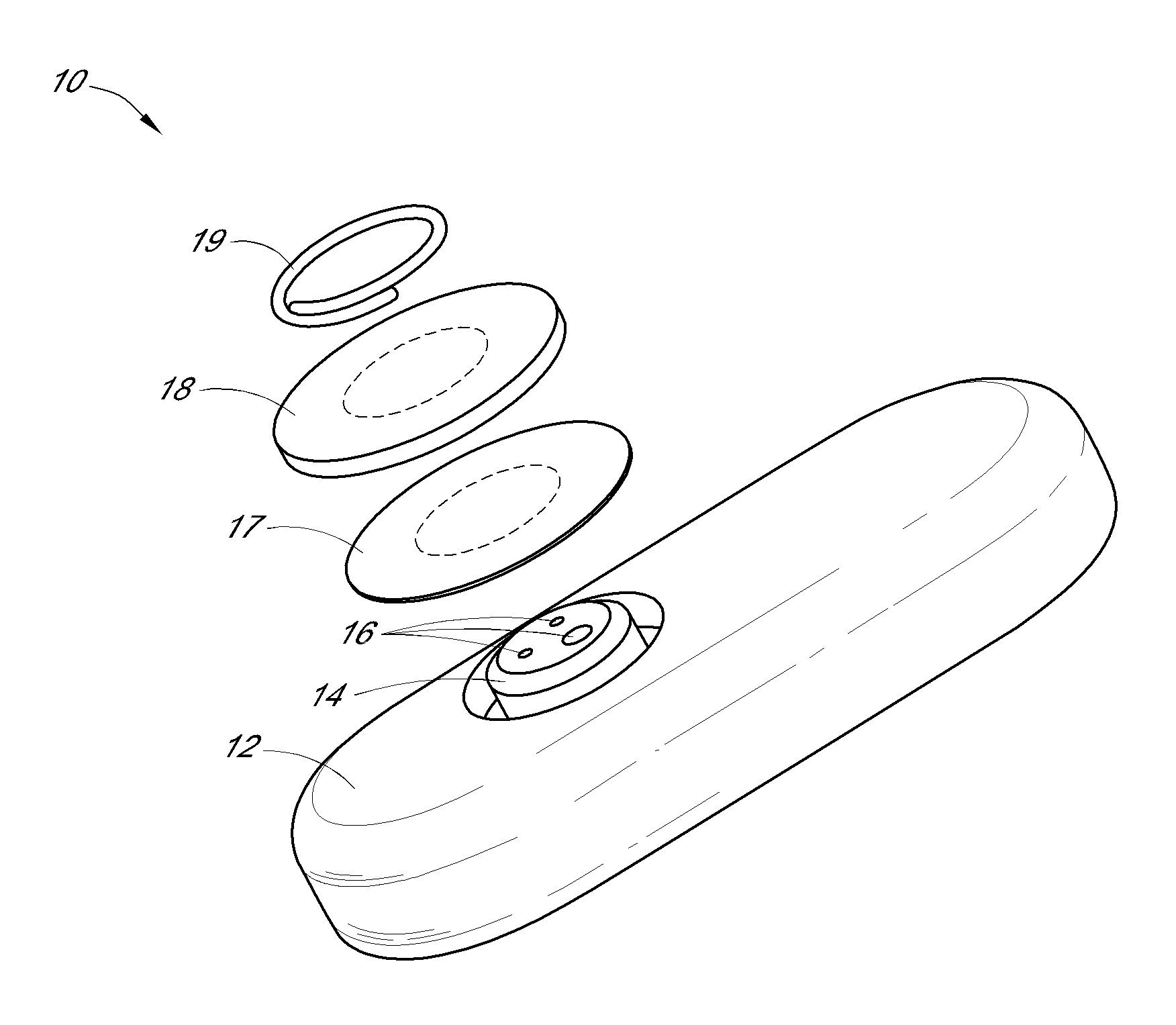
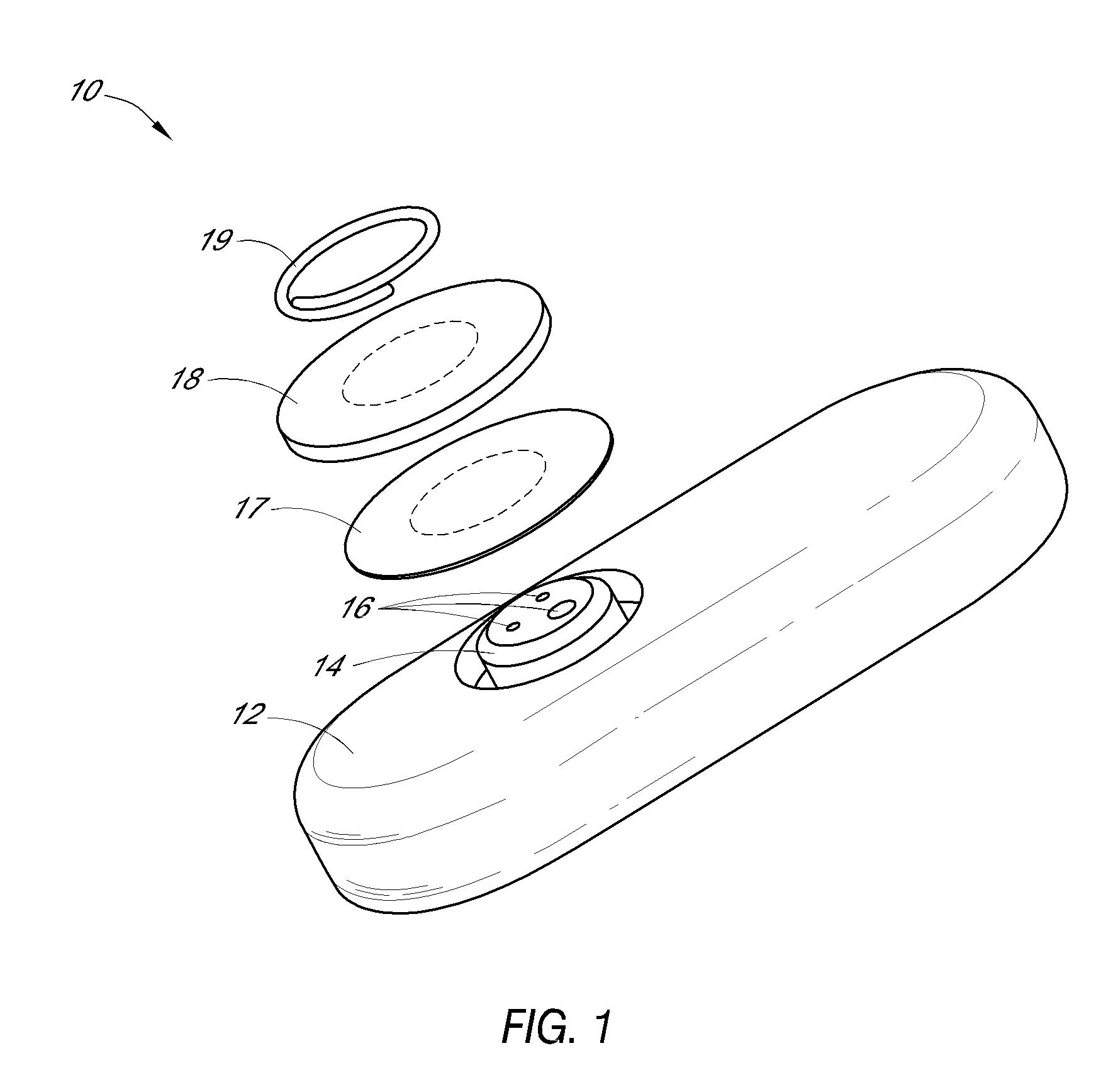
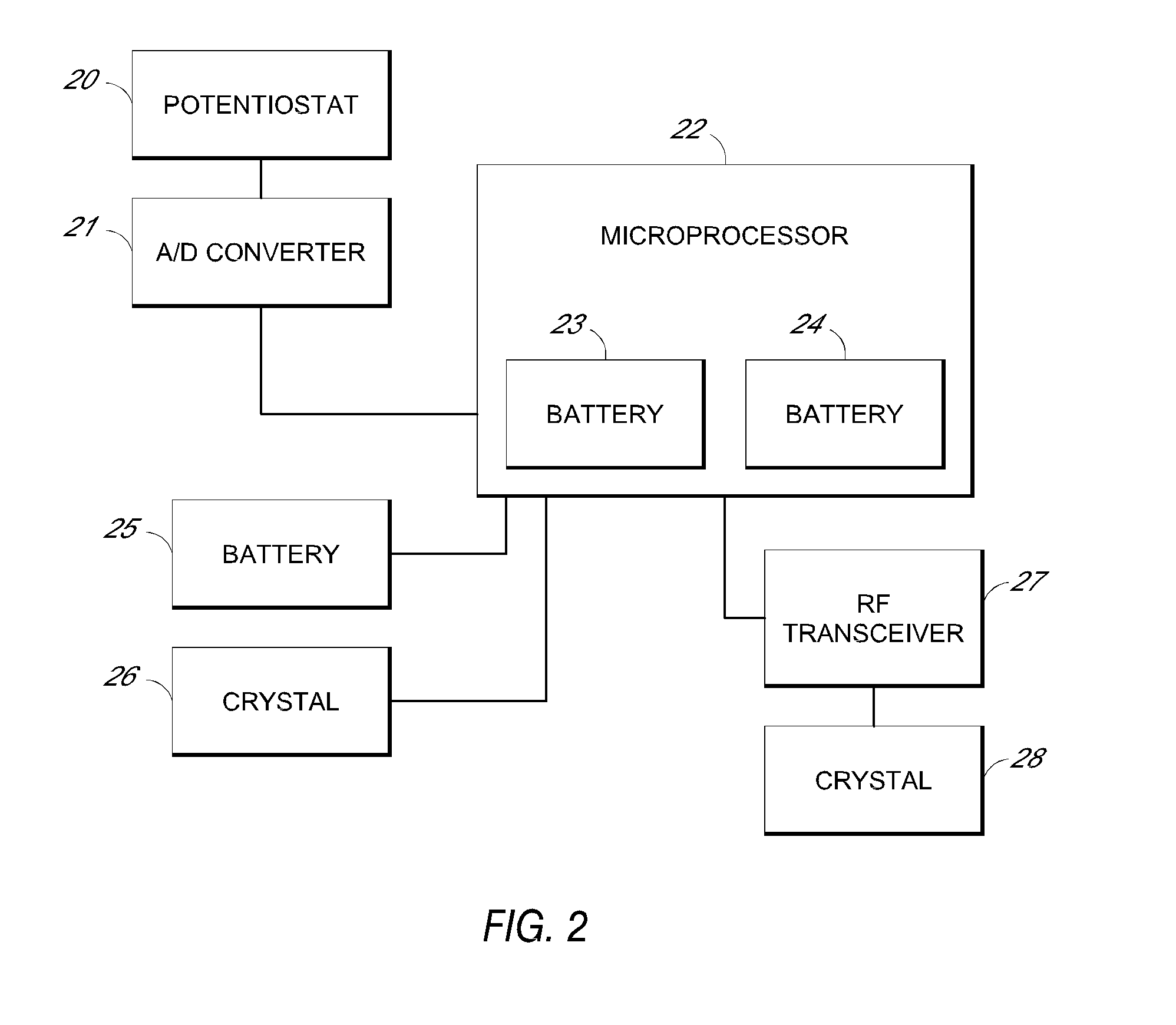
[0299]FIG. 4A illustrates a first embodiment wherein the receiver shows a numeric representation of the estimated analyte value on its user interface, which is described in more detail elsewhere herein.
second embodiment
[0300]FIG. 4B illustrates a second embodiment wherein the receiver shows an estimated glucose value and one hour of historical trend data on its user interface, which is described in more detail elsewhere herein.
third embodiment
[0301]FIG. 4C illustrates a third embodiment wherein the receiver shows an estimated glucose value and three hours of historical trend data on its user interface, which is described in more detail elsewhere herein.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com