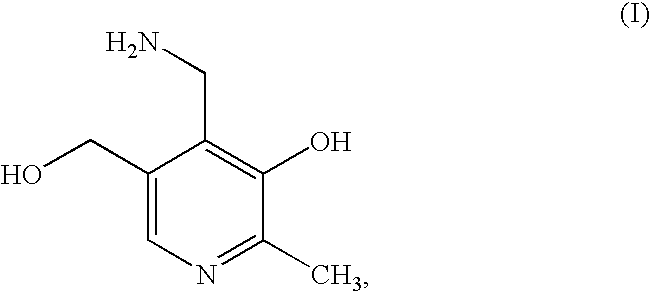
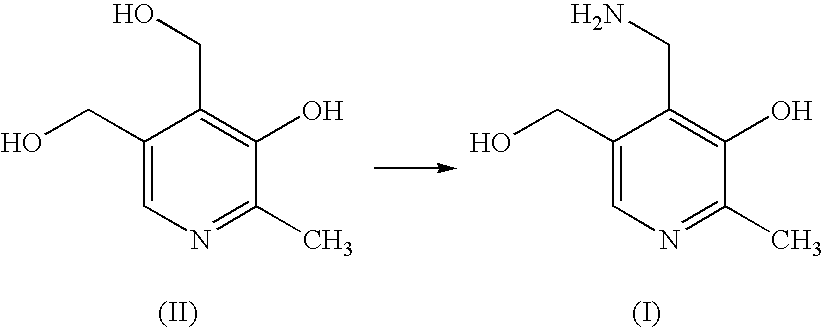
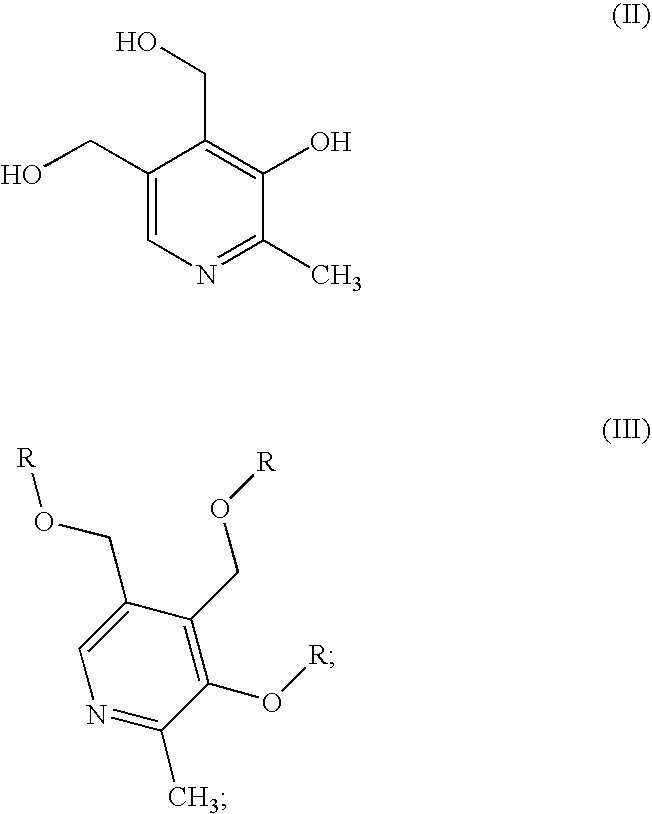
Methods for the synthesis of pyridoxamine
a technology of pyridoxine and pyridoxamine, which is applied in the direction of biocide, drug composition, metabolic disorder, etc., can solve the problems of non-selective oxidation of the second hydroxymethyl group at the 5 and the problem of pyridoxine oxidation is problemati
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Acetylation of Pyridoxine (II)
[0166]
Synthesis
[0167]A 160 L reactor was inertised and charged with pyridoxine hydrochloride (II) (15.915 kg) in TBME (46 L). Acetic anhydride (3.25 eq., 24.5 L) was added and the suspension was heated to 50° C. Triethylamine (4 eq., 43 L) was added slowly over a period of 1 hour. During the addition of triethylamine, the inner temperature increased to 65° C. The mixture was stirred at reflux temperature for 1.5 hours. The reaction was completed when TLC analysis showed complete acetylation of pyridoxine.
Workup
[0168]The reaction was cooled to 10° C., filtered, and the precipitate was washed with TBME (3×18 L). The combined organic layers were stirred for 25 minutes with saturated NaHCO3 (sat. 40 L soln., pH 6–7). The organic and aqueous layers were separated, with the aqueous layer extracted again with TBME (20 L). The combined organic layers were extracted with saturated NaHCO3 (40 L, pH 9). Analysis by 1H NMR showed no residual acetic anhydride. The o...
example 2
Preparation of Phthalimido-Derivative (D)
[0173]
[0174]A 160 L reactor was inertised and charged with the product from Example 1 in TBME (22.367 kg (C), 121 L). The solution was concentrated to a volume of about 40 L, under reduced pressure and a jacket temperature of 60° C. To the reduced volume was added DMSO (50 L). The distillation of TBME was continued until 1H NMR showed 5.4 mol % of residual TBME (compared to amount of (C); limit: 10 mol %). To this was added DMSO (7.5 L), phthalimide potassium salt (1.3 eq., 18.211 kg), and THF (9 L) and the mixture was stirred at 40° C. for 34.hr. At this point 1H NMR showed 98.2% conversion to (D).
[0175]The reaction mixture was cooled to 20° C. and divided into two portions (2×50 L). To each portion THF (45 L and 44.5 L), water (11 L and 12 L), and half-saturated NaCl (25 L and 23.5 L) were added, and the resulting aqueous and organic layers were separated. The combined aqueous layers were extracted with THF (3×20 L). The combined organic la...
example 3
Preparation of Methylamide Derivative of Pyridoxamine (V)
[0181]
[0182]A 160 L reactor was inertised and charged with the product of Example 2 in THF (26.216 kg (D) in 133 L). The solution was heated to 48–50° C. and propylamine (4 eq., 25.1 L) was added over a period of 33 minutes (temperature increased to 55° C. during addition). The reaction mixture (solution) was stirred at 65° C. for 15 h. A suspension formed. 1H NMR showed conversion >99% (compared to intermediate with signal at δ=5.23 ppm, 2H).
[0183]The suspension was cooled to 5° C. over a period of 1 h and stirred at this temperature for 40 min. The suspension was filtered over a wet celite bed (2.403 kg Celite, 10 L THF) to give a clear orange mother liquid. The filtration was quite slow (˜7.5 h). The filter cake (crude 1#1—phthalic acid dipropylamide) was washed with cold (5° C.) THF (2×48 L). The combined organic layers (215 L) were concentrated to a volume of about 30 L at a 60° C. jacket temperature under reduced pressur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com