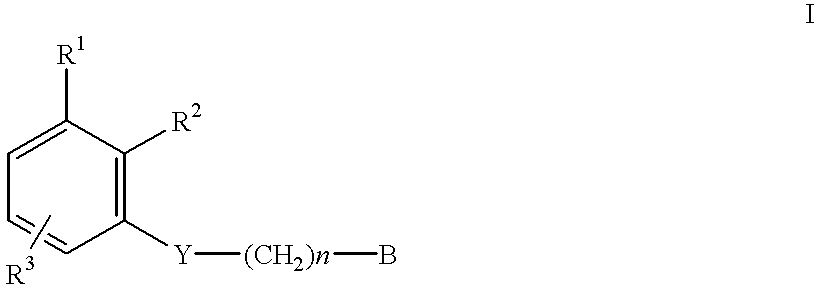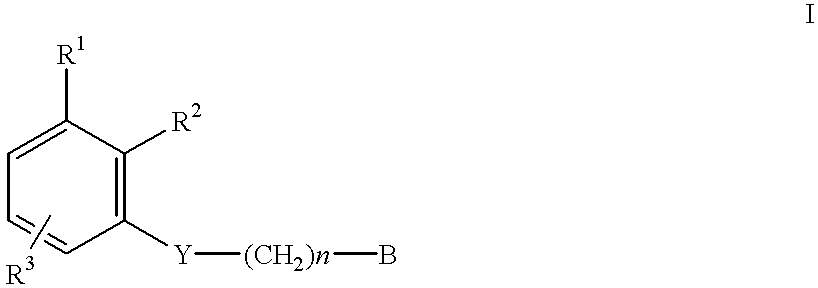Amidino derivatives and their use as thrombin inhibitors
a technology of amidino derivatives and inhibitors, which is applied in the direction of phosphorous compound active ingredients, drug compositions, group 5/15 element organic compounds, etc., can solve problems such as thromboembolic diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N-{3-[2-(4-Aminoiminomethylphenyl)ethoxy]phenyl}benzenesulfonamide.times.HCl
(i) t-Butyloxycarbonylamino-3-hydroxybenzene
Amino-3-hydroxybenzene (5.46 g; 50 mmol) was dissolved in THF (50 mL) and di-t-butyl dicarbonate (12.0 g; 55 mmol) was added at room temperature. The solution was heated for 2 hours at 60.degree. C., the solvent was evaporated and the residue was dissolved in EtOAc (150 mL). The EtOAc-phase was washed with 2.times.20 mL of 1M KHSO.sub.4, 1.times.20 mL water, 1.times.20 mL brine and then dried (MgSO.sub.4). The solvent was evaporated to give 11.74 g of a colourless oil which was crystallised from CH.sub.2 Cl.sub.2 :MeOH:light petroleum to give 9.1 g (87%) of the sub-title compound as white crystals.
.sup.1 H-NMR (400 MHz; CDCl.sub.3): .delta.7.10-7.15 (bs, 1H), 7.11 (t, 1H), 6.72 (dd, 1H), 6.53 (dd, 1H), 6.50 (bs, 1H), 5.57 (apparent bs, 1H), 1.52 (s, 9H) .sup.13 C-NMR (100 MHz; CDCl.sub.3): .delta.156.4, 152.8, 139.4, 129.9, 110.7, 110.2, 105.9, 80.8, 28.3
(ii) t-But...
example 2
Benzenesulfoniacid-{3-[2-(4-aminoiminomethylphenyl)ethoxy]-5-methyl}-phenyl ester.times.HCl
(i) Benzenesulfonic acid-(3-hydroxy-5-methyl)phenyl ester
Benzenesulfonyl chloride (6.36 g; 36 mmol) was added to a well stirred mixture of 3,5-dihydroxytoluene.times.H.sub.2 O (4.26 g; 30 mmol), saturated aqueous NaHCO.sub.3 (70 mL) and Et.sub.2 O (50 mL) and the mixture was stirred at room temperature for 19 hours. Et.sub.2 O (50 mL) was added and the organic layer was separated, collected and evaporated to give 7.05 g of a powder. The crude material was recrystallized from EtOAc:heptane (30 mL:300 mL) to give 4.36 g (55%) of the sub-title compound.
FAB-MS 265 (M+1).sup.+
.sup.1 H-NMR (400 MHz; CDCl.sub.3): .delta.7.86 (d, 2H), 7.67 (tt, 1H), 7.53 (t, 2H), 6.54 (bs, 1H), 6.38 (bs, 1H), 6.31 (t, 1H), 5.02 (s, 1H, OH), 2.21 (s, 3H)
(ii) Benzenesulfonic acid-{3-[2-(4-cyanophenyl)ethoxy]-5-methyl}phenyl ester
Diethylazodicarboxylate (1.74 g; 10 mmol) was added, over 5 minutes, to a stirred solution o...
example 3
N-{3-[2-(4-Aminoiminomethylphenyl)ethoxyl]phenyl}-2-chlorobenzenesulfonamide.times.HOAc
(i) Nitro-3-[2-(4-cyanophenyl)ethoxy]benzene
Triphenylphosphine (11.3 g; 43.1 mmol) and diethylazodicarboxylate (7.5 g; 43 mmol) were dissolved in THF (250 mL) under nitrogen at 0.degree. C. After 5 minutes, 3-nitrophenol (5.00 g; 35.9 mmol) and 2-(4-cyanophenyl)ethanol (6.3 g; 43 mmol) were added. The cooling bath was removed and the mixture stirred for 2 days at room temperature. A new batch was prepared as above and the two were combined before work-up. Water was added and the THF was evaporated. The mixture was extracted with EtOAc, and the organic phase was washed with aqueous 0.2M NaOH and brine, dried (Na.sub.2 SO.sub.4) and the solvent removed in vacuo. Purification by flash chromatography (SiO.sub.2 ; toluene) and recrystallization from CH.sub.2 Cl.sub.2 :EtOH afforded 3.07 g (32%) of the sub-title compound.
.sup.1 H NMR (500 MHz; CDCl.sub.3): .delta.7.82 (dd, 1H), 7.61-7.71 (several peaks,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| ionic strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com