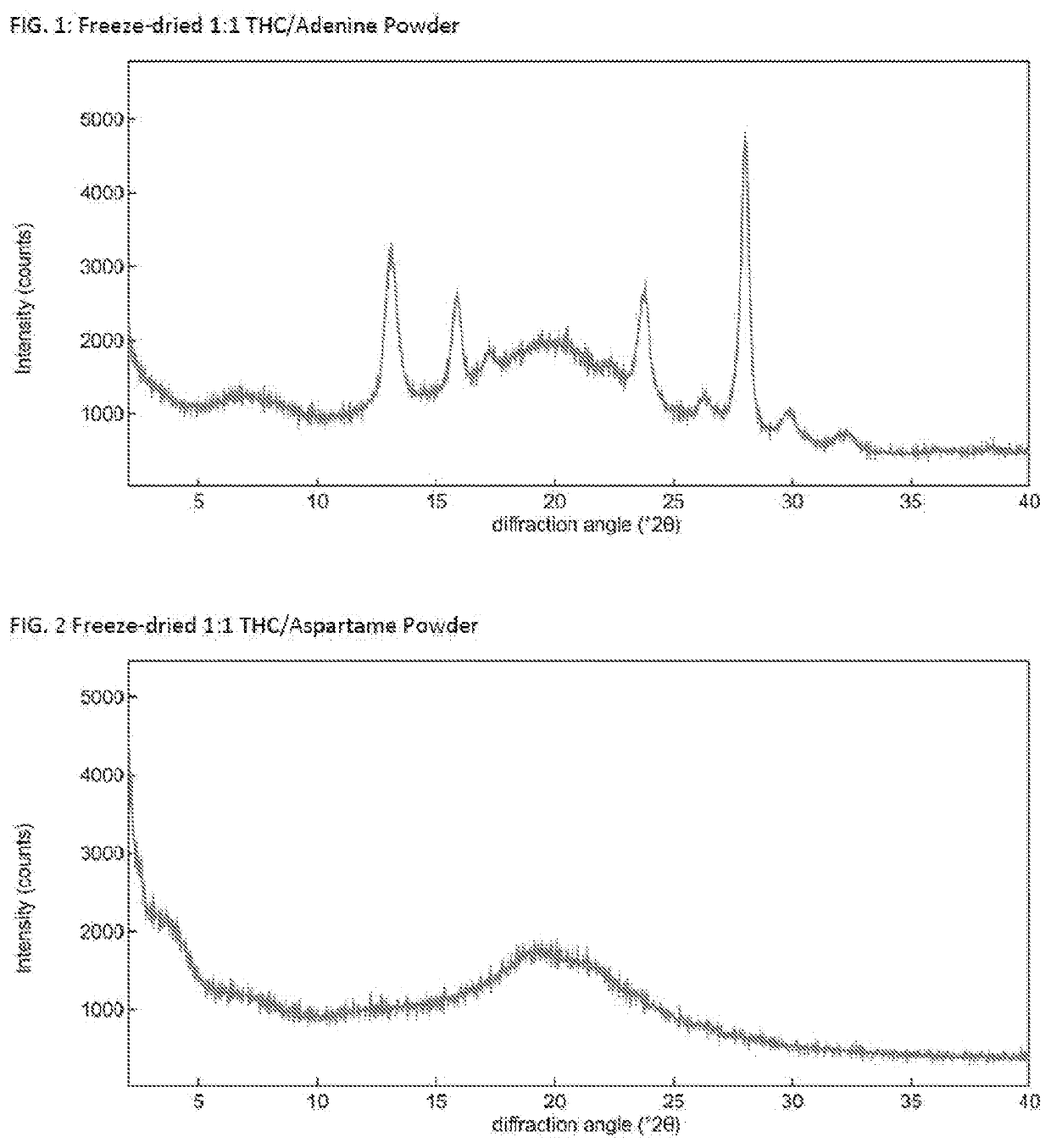
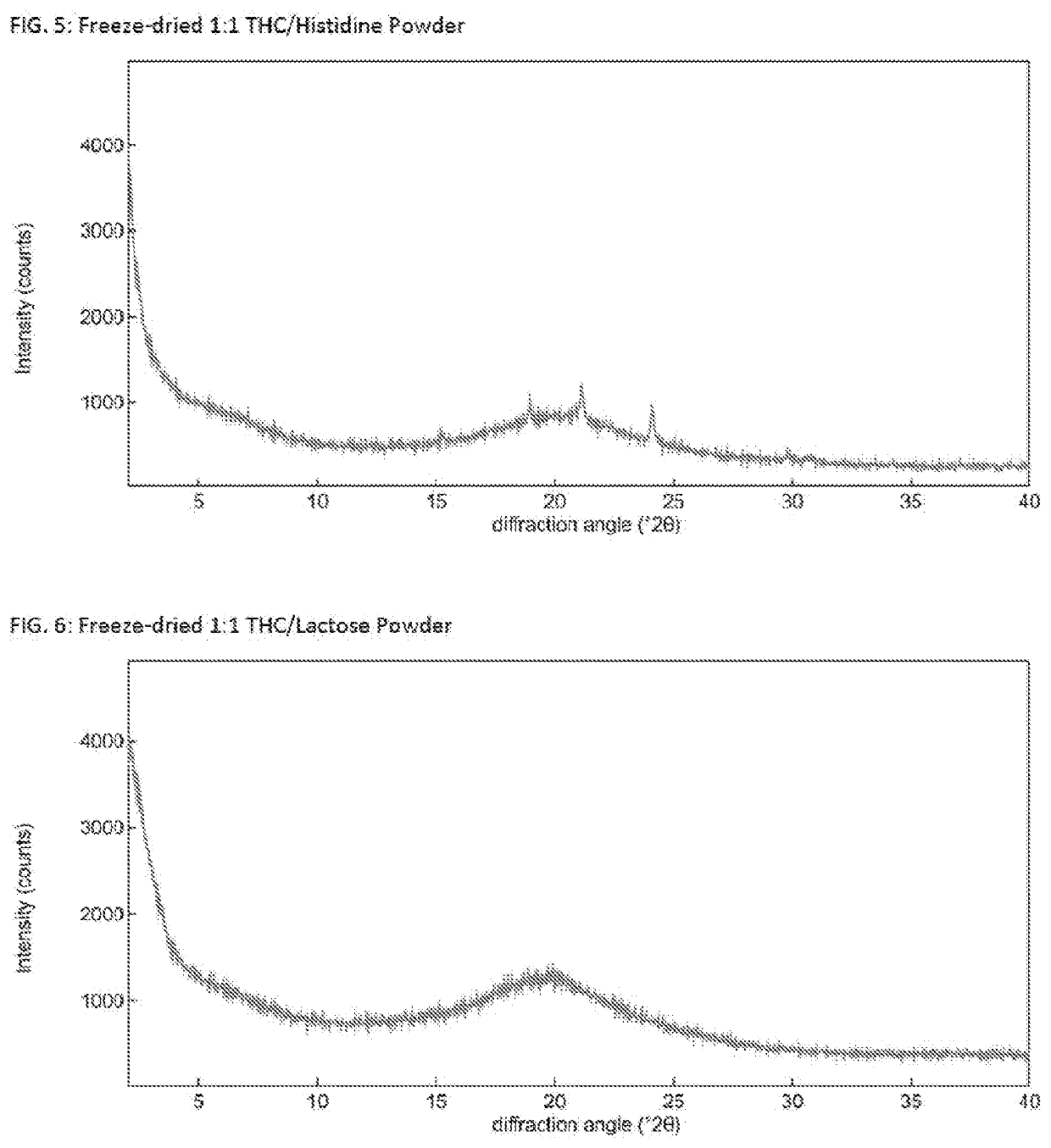
Solid delta9-tetrahydrocannabinol (delta9-thc) compositions
a technology of delta9 and tetrahydrocannabinol, which is applied in the direction of lyophilised delivery, medical preparations, granular delivery, etc., can solve the problems of inability to store the pharmaceutical formulation in the refrigerator, the inability to manufacture, and the inability to adapt to the use of sup>9/sup>-th
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
up>9-THC Compositions by Freeze-Drying (Lyophilization)
[0095]A commercially available Δ9-THC-acetonitrile solution (Cayman Chemical), approximately 50 mg Δ9-THC per mL, was used. The actual concentration was determined gravimetrically by taring a vial and recording its weight, adding 1 mL of solution, evaporating the solution, vacuum drying the sample at room temperature for 12 hours and reweighing the vial. The actual concentration was determined to be 51 mg / mL. Into 1 dram vials, 300 μl of the Δ9-THC acetonitrile solution was dispensed and allowed to evaporate at room temperature resulting in each vial containing ca. 15.3 mg of Δ9-THC.
[0096]Δ9-THC (ca. 15.3 mg) was dissolved in dioxane (3 mL solvent used) and transferred into a 25 mL round bottom flask. The powder former (amount based on 1:1, 1:2 or 1:4 molar Δ9-THC:powder former ratio) was dissolved in H2O (2 mL) and, if needed, dioxane (2 mL) was added to form a solution. The powder former solution was added to the Δ9-THC soluti...
example 2
up>9-THC Compositions by Evaporation
[0100]Δ9-THC (ca. 15.3 mg, prepared as in Example 1) was dissolved in methanol (MeOH) (1 mL). A powder former (amount based on a 1:1 Δ9-THC:powder former molar ratio) was added to the solution. If needed, additional MeOH (1-3 mL) was added until solids dissolved completely. The vial was left uncapped for fast evaporation (FE) at ambient temperature. The powder formers used were aspartame, caffeine, histidine, lactose and L-pipecolic acid. The Δ9-THC / histidine mixture resulted in a gel after evaporation. The other Δ9-THC / powder former mixtures were flowable powders and remained flowable powders when left at ambient temperature. The XRPD patterns were obtained for the Δ9-THC / aspartame powder (FIG. 21), Δ9-THC / caffeine powder (FIG. 22), and Δ9-THC / L-pipecolic acid powder (FIG. 23). Each XRPD pattern showed the presence of some crystalline powder former and an amorphous halo.
example 3
up>9-THC Compositions by Physical Mixing
[0101]Δ9-THC (ca. 15.3 mg, prepared as in Example 1) and a powder former (amount based on 1:1 Δ9-THC:powder former molar ratio) were placed in a 1 dram vial. The solids were mixed with a glass stir rod and metal spatula. The powder formers used were aspartame, caffeine, histidine, lactose and L-pipecolic acid. The resulting material was a powder. The resulting materials were flowable powders and remained flowable powders when left at ambient temperature. The Δ9-THC / histidine mixture resulted in a tacky solid. The XRPD patterns were obtained for the Δ9-THC / aspartame powder (FIG. 24), the Δ9-THC / caffeine powder (FIG. 25), the Δ9-THC / lactose powder (FIG. 26), and the Δ9-THC / L-pipecolic acid powder (FIG. 27). The XRPD patterns showed the presence of some crystalline powder former and an amorphous halo.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| beam size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com