Protocol for minimizing toxicity of combination dosages and imaging agent for verification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Administration of Conjugated SN-38
[0099]SN-38 is the topoisomerase I inhibitor that is the active drug released from the prodrug, irinotecan. Conjugates of SN-38 are described in WO 2015 / 051307. Two different conjugates of SN-38 were prepared: PLX038 and PLX038A. These conjugates couple the drug releasably to a four-armed PEG of 40 kD through a linker that effects release by β-elimination. The structure of PLX038 and PLX038A is shown below wherein “Mod” is CN in PLX038, and methyl sulfonyl in PLX038A.
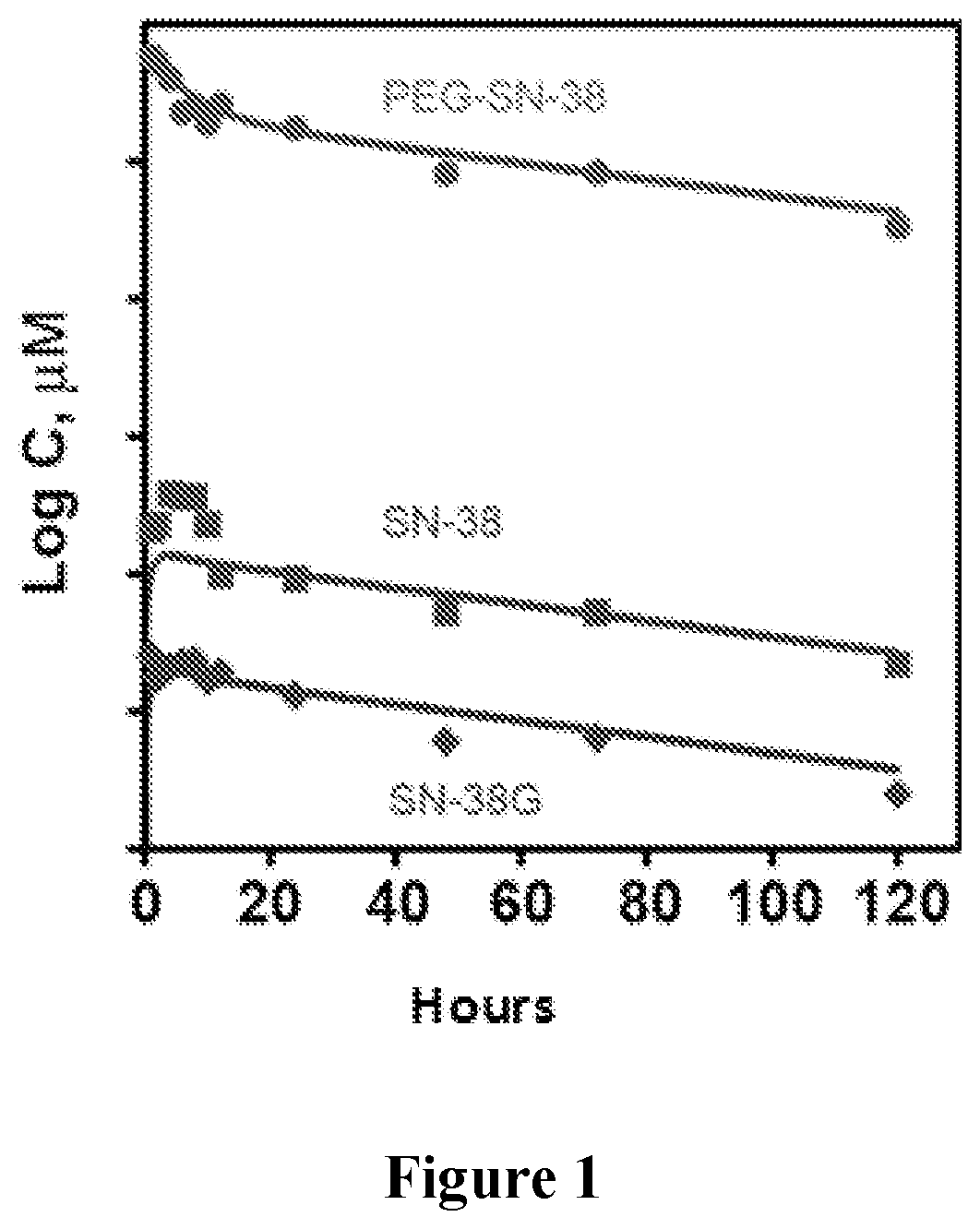
[0100]Six rats bearing HT29 tumor xenografts were injected with 200 mg / kg of PLX038 and the concentration in plasma and tumor of the conjugate and released drug as well as its glucuronide (SN-38G) were followed by HPLC with fluorescence monitoring As shown in FIG. 1, the half-life of PLX038 in the systemic circulation is about 50 hours. The conjugate and the free drug as well as SN-38G show similar half-lives.
[0101]As shown in FIG. 2, the efficacy of a non-toxic dose of 20 nmol / kg of SN...
example 2
Suggested Human Protocol
[0103]A dosing schedule in humans for a combination of PLX038 and a second drug (e.g., a PARP inhibitor) administered systemically is proposed wherein PLX038 is administered on day 1 whereby the conjugate accumulates in the tumor and releases the free drug. The conjugate and the free drug are concomitantly cleared from the system. After two half-lives of systemic clearance or 10 days, systemic PLX038 is reduced to 25% of its original concentration, which lies below its minimal effective and toxic levels. At this time the second drug, which is synergistic with SN-38 is administered orally for 20 days.
[0104]As shown in FIG. 4, the EPR effect concentrates PLX038 in the tumor (dotted line), while the systemic PLX038 (solid line) is sufficiently low that any toxic effect is only to a second drug, which is administered as shown at 10 days. At that time, the concentration of the conjugate in the tumor is still above the minimum effective level but below the toxic le...
example 3
Design of a Mouse Model
[0105]Because most xenograft tumor models use mice as hosts, it is desirable to adapt the protocols of the present invention to testing in mice. Adaptation is needed because the half-life of the PLX038 conjugate in the mouse is only about 24 hours, whereas in the rat it is about 48 hours and in humans about 6 hours. Because the more rapid elimination of PLX038 in mice occurs before substantial amounts of the SN-38 are released, a different conjugate of SN-38, PLX038A that has a higher cleavage rate, is used in murine experiments.
[0106]Linker cleavage is species independent. While 32% of PLX038 is converted to SN-38 over one half-life of the conjugate in humans, only 12% is converted in the rat and 6% in the mouse. For PLX038A, the cleavage half-life is 70 hours and 26% conversion to SN-38 over one half-life of the conjugate in the mouse occurs. This conjugate also can be administered intraperitoneally (IP) in mice with 100% bioavailability.
[0107]However, in mi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydrodynamic radius | aaaaa | aaaaa |
| Nanoscale particle size | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com