Toxoplasma gondii vaccines and their use
a technology of toxoplasma gondii and vaccines, which is applied in the field of intracellular parasites, can solve the problems of toxic or hypersensitivity, inability to eliminate latent parasites, and cyst forms that cannot be eliminated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Adjuvanted Multi-Epitope Vaccines Protect HLA-A*1101 Transgenic Mice Against Toxoplasma Gondii
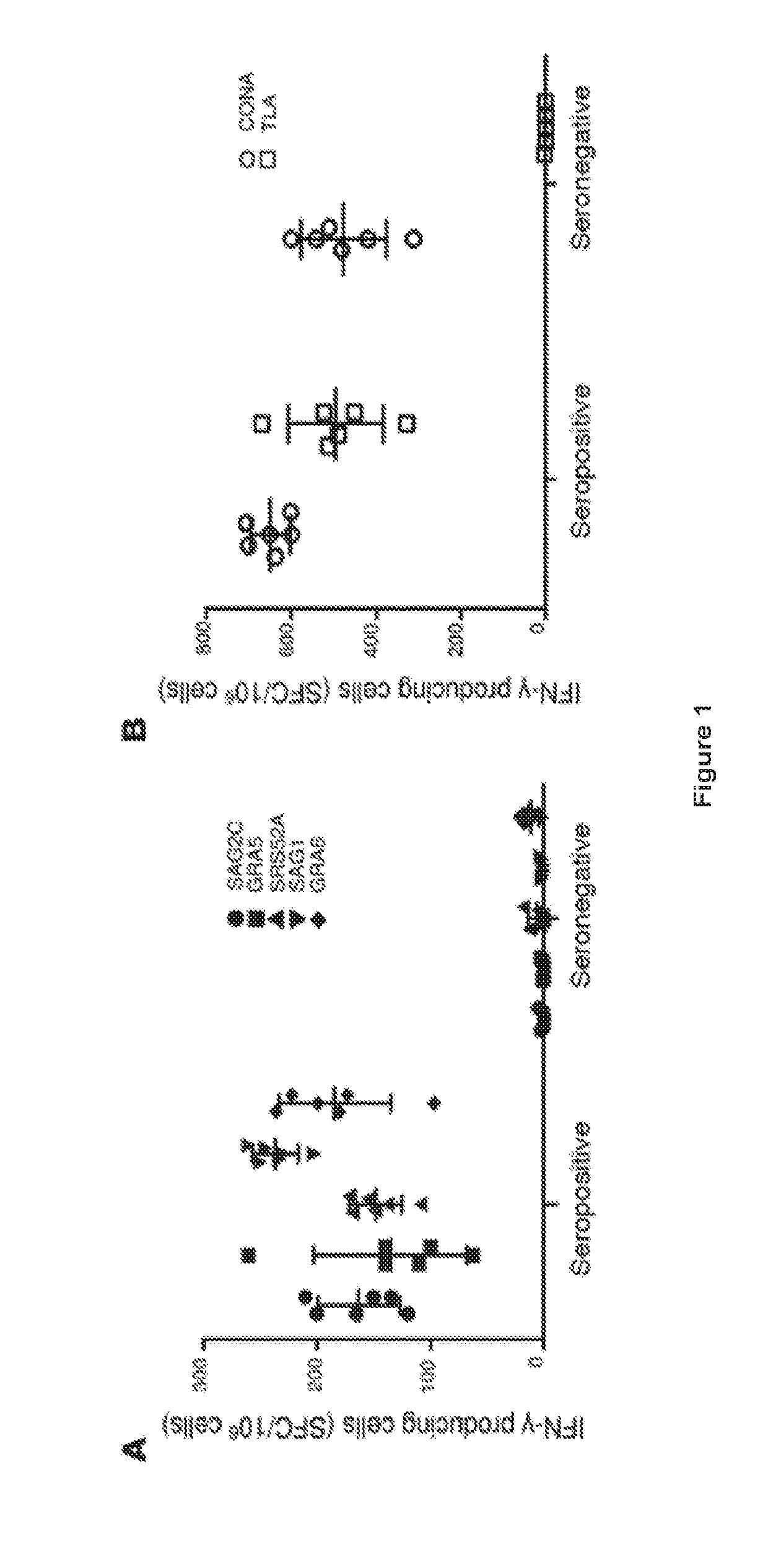
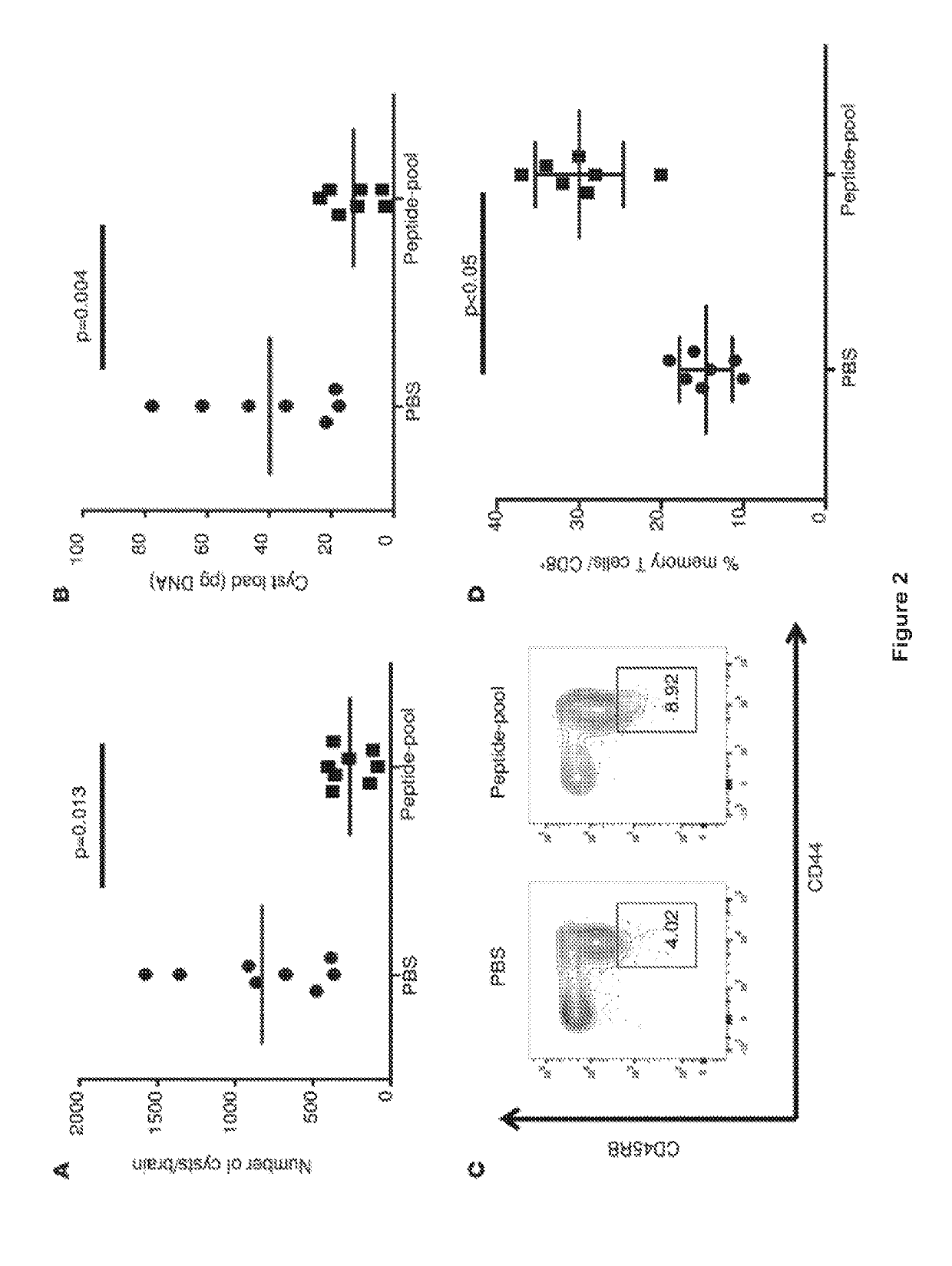
[0076]We created and tested multi-epitope DNA or protein vaccines with TLR4 ligand emulsion adjuvant (gluco glucopyranosyl lipid adjuvant in a stable emulsion (GLA-SE)) for their ability to protect against Toxoplasma gondii in HLA transgenic mice. Our constructs each included five of down selected CD8+ T cell eliciting epitopes, a universal CD4+ helper T lymphocyte epitope (PADRE), a secretory signal, all arranged to maximize MHC Class I presentation. Their capacity to elicit immune and protective responses was studied using immunization of HLA-A*1101 transgenic mice. These multi-epitope vaccines increased memory CD8+T cells that produced IFN-γ and protected mice against parasite burden when challenged with T. gondii. Endocytosis of emulsion-trapped protein and cross presentation of the antigens may account for the immunogenicity of our adjuvanted protein. This work demonstrates a novel a...
example 2
Novel Protein Nanovaccine Confers Robust Immunity Against Toxoplasma
[0110]We designed and produced a self-assembling protein nanoparticle (SAPN) containing five CD8+ HLA-A03-11 supertypes restricted epitopes from antigens expressed during Toxoplasma's lifecycle, PADRE which is a CD4+ T cell, universal epitope, and flagellin as scaffold and TLR5 agonist. These epitopes were separated by N / KAAA (SEQ ID NO: 21 & 22) spacers and optimized for proteasomal cleavage. SAPN-GLA-SE was evaluated for its efficacy in inducing IFN-γ and protection against T. gondii in mice with an HLA-A*1101 transgene. In our data, immunization with SAPN adjuvanted with TLR4 ligand-emulsion (GLA-SE), activated CD8+ T cells to produce IFN-γ. SAPN-GLA-SE also protected against subsequent challenge with type II parasites in mice with an HLA-A*1101 transgene. Hence, combining CD8+ T cell-eliciting peptides and PADRE into a multi-epitope within a single self-assembling protein, administered with adjuvant GLA-SE, lea...
example 3
Novel, Immunogenic, Self-Replicating RNA Nanoparticle, Platform Encoding Immunosense Designed Toxoplasma Peptides is A Vaccine That Protects HLA-A*1101 Transgenic Mice
[0133]We designed and produced a self-replicating RNA nanoparticle displaying peptide epitopes that induces protective CD8+ and CD4+ T cells against T. gondii infection. These RNA replicons are composed of Venezuelan Equine Encephalitis (VEE) alphavirus backbone, 5 CD8+ HLA-A03-1101 supertype-restricted epitopes from antigens expressed during the life cycle of Toxoplasma, and a universal CD4+ T cell epitope (PADRE). All are encapsulated in lipid nanoparticles and evaluated for their immunogenic and protective potential against T. gondii in HLA-A*1101 transgenic mice. Administered without the need for an adjuvant, the self-replicon nanoparticles elicit T cells producing IFN-γ and protect mice against parasite burden when challenged with type II T. gondii strain. Thus, this work demonstrates that RNA replicon nanoparticl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com