17-hydroxyprogesterone ester-containing oral compositions and related methods
a technology of progesterone and ester, which is applied in the direction of medicine preparations, powder delivery, metabolism disorders, etc., can solve the problems of increasing the distress and/or anxiety of patients, increasing the time and cost of ptb related intensive care, and neonatal morbidity and mortality, and achieves the effect of effective oral delivery, increased w/w loading of ester, and effective bioavailability of ester 17hp
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
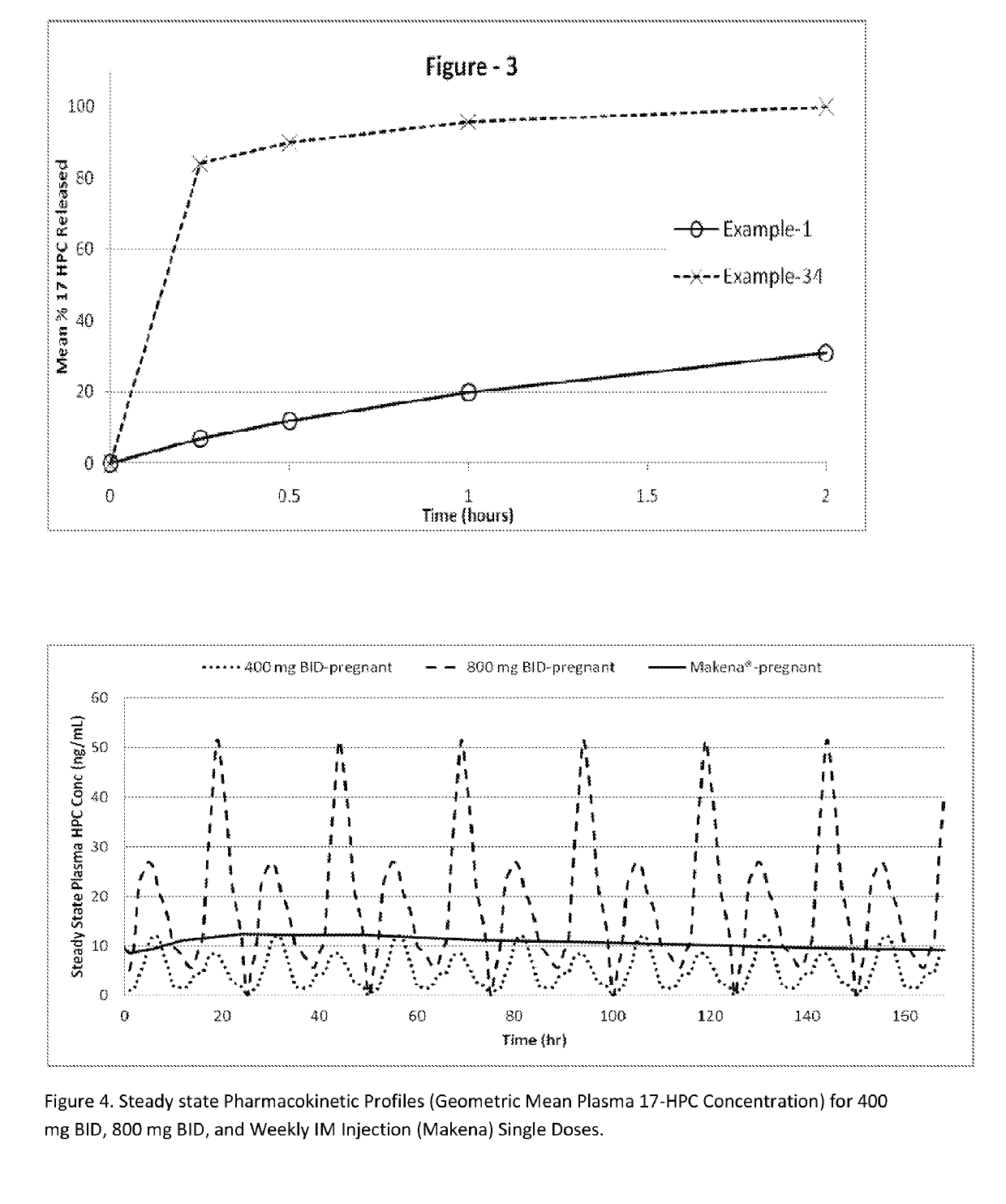
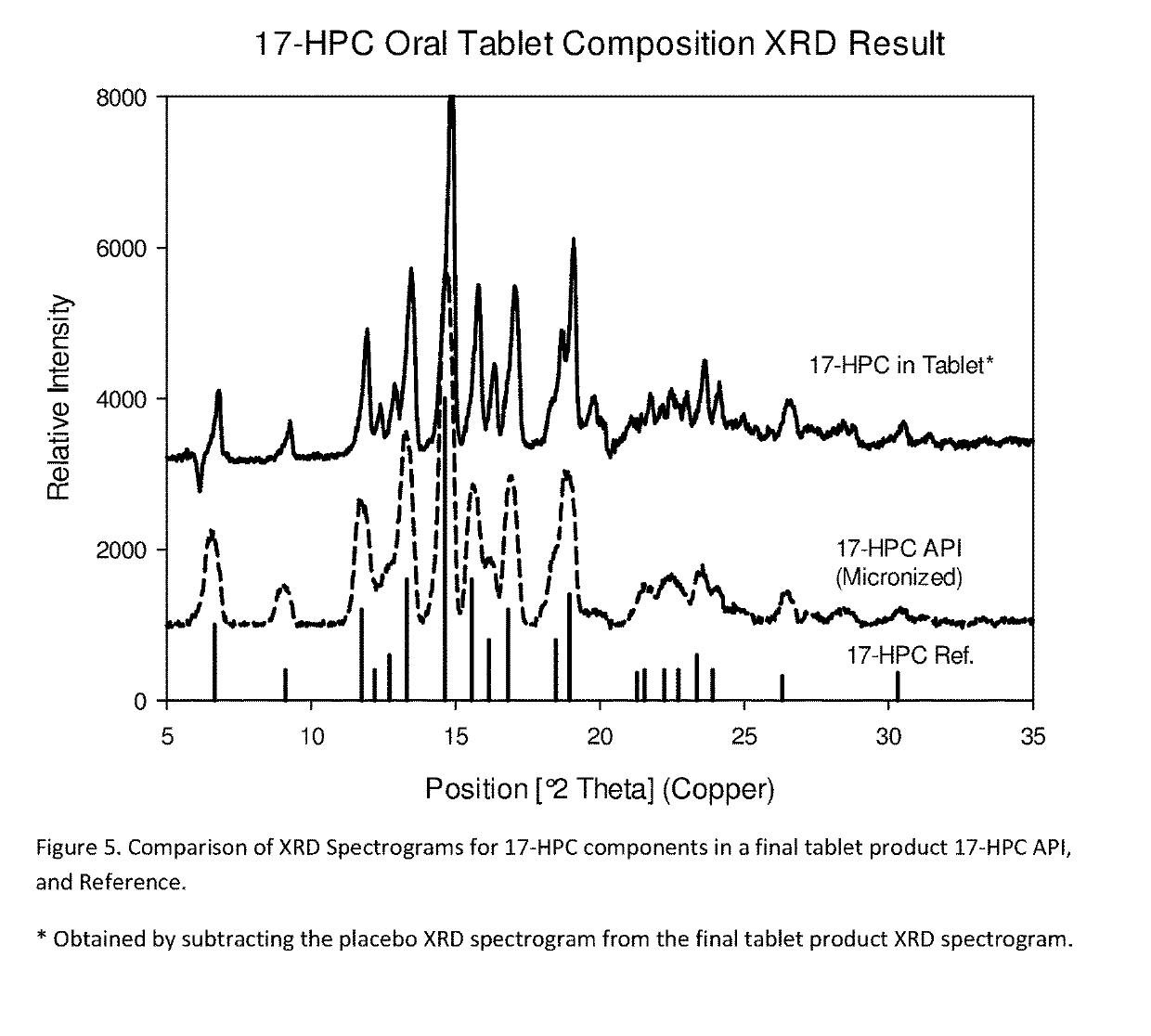
[0306]The following examples are provided to promote a more clear understanding of certain embodiments of the present invention, and are in no way meant as a limitation thereon. Unless otherwise specified or mentioned, all the compositions provided in the examples are with respect to % w / w of the final composition. Note that with the exception of the compositions listed in Examples 1, 7, 10, 17 and 36, the 17-hydroxyprogesterone caproate of all other example compositions can be in either treated (milled, micronized, or nanosized) or untreated form. The 17-hydroxyprogesterone Caproate in compositions 1, 7, 10, 17 and 36 are untreated for size reduction (i.e., unmilled, non-micronized, un-micronized or non-nanosized), and have an average particle size greater than 50 micrometers. The dosage forms of corresponding Examples were tested for release of the 17-hydroxyprogesterone caproate using a USP Type II apparatus, 50 rpm in 900 mL of simulated intestinal fluid having 0.5% w / w sodium l...
examples 1-6
17-Hydroxyprogesterone Caproate Compositions
[0307]17-hydroxyprogesterone caproate compositions as recited in Examples 1 through 6 are prepared by using the respective components shown in Table I. Example 1 is the untreated crystalline form of 17-hydroxyprogesterone caproate filled into hard gelatin capsule. Example 2 is micronized 17-hydroxyprogesterone caproate without a carrier filled into hard gelatin capsule. Examples 3-6, are prepared as follows: The required quantities of each of the components of the respective composition, except 17-hydroxyprogesterone caproate are taken in a clean stainless steel container and mixed at about 50° C. to 70° C. using a stirrer. A molten clear-to-hazy mixture is obtained. The required amount of the 17-hydroxyprogesterone caproate is added to the clear-to-hazy mixture and stirred to form a homogenous liquid mixture. A predetermined weight of the resulting liquid mixture is disposed into appropriate size capsules according to the 17-hydroxyproges...
examples 7-10
17-Hydroxyprogesterone Caproate Compositions
[0310]17-hydroxyprogesterone caproate compositions of Examples 7 through 10 can be prepared by using the ingredients shown in Table II and attain the release performance indicated.
TABLE IIExample No.78910IngredientsComposition in % w / w.17-hydroxyprogesterone caproate90-99——90-99(particle size > 50 μm)17-hydroxyprogesterone caproate—70-80——micronized*17-hydroxyprogesterone caproate——70-80—(milled)Lactose 1-10 1-20 1-20 30Povidone K303-63-63-63-6Organic granulating solvent—0 or0 orq.s***(example, alcohol)**q.s***q.s***—% release in 60 mins>50>50>30*may be substituted with nanomilled or nanosized 17-hydroxyprogesterone caproate.**removed substantially during drying process***Quantity sufficient for wet granulation process or for in situ formation / precipitation of fast releasing solid 17-hydroxyprogesterone caproate
According to one aspect, the formulations (e.g., unit dosage forms) in the above table have from about 100 mg to 495 mg of 17HPC. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com