Sequence variant analysis of cell-free DNA for cancer screening
a cell-free dna and sequence variant technology, applied in the field of sequence variant analysis of cell-free dna for cancer screening, can solve the problems of increased likelihood that the bodily fluid might contain tumor-derived dna, sequencing errors that can occur and may be misinterpreted, and subjects carrying hepatitis b or c virus have an increased risk of developing hepatocellular carcinoma. , to achieve the effect of accurate parameter and level of tumor heterogeneity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
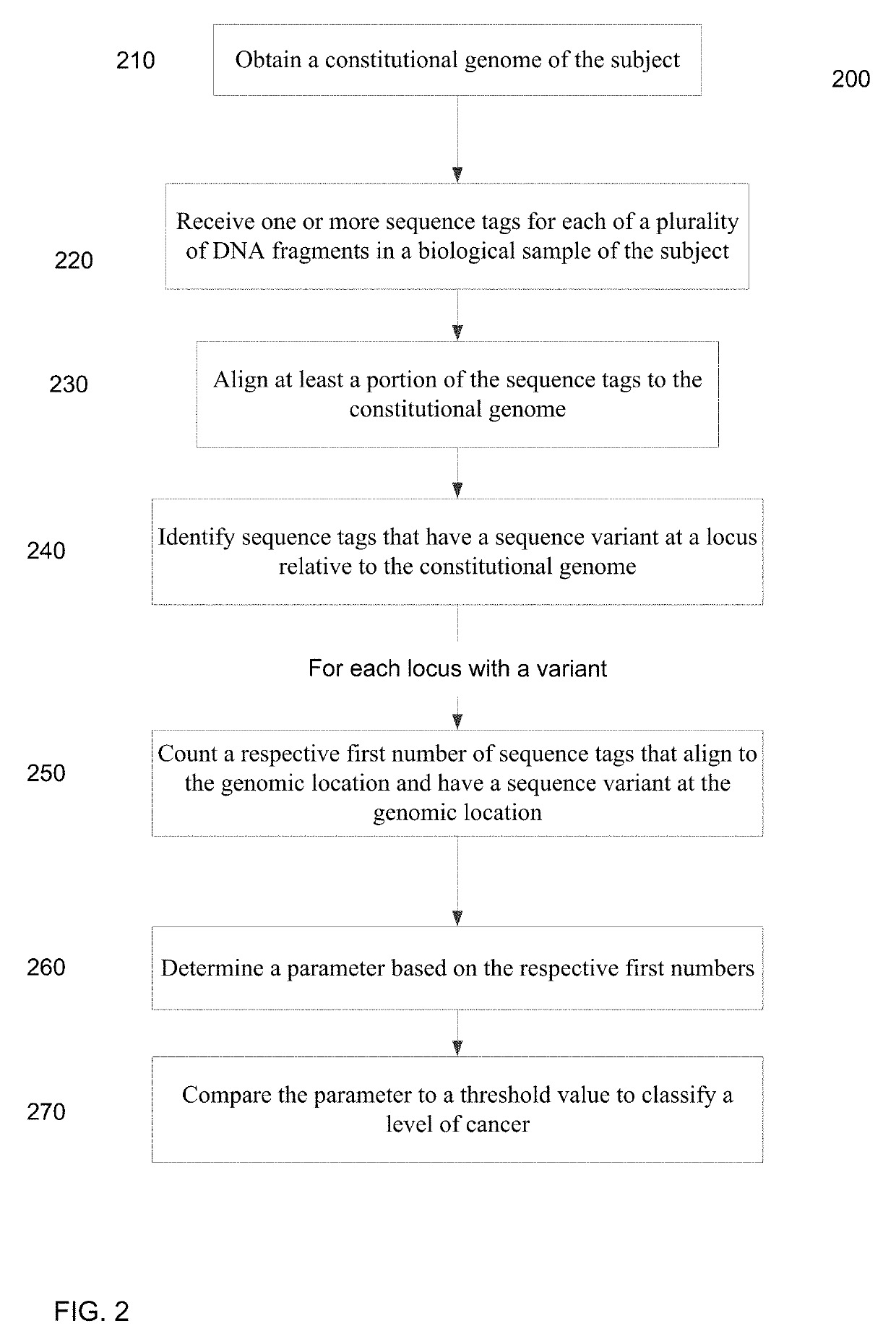
[0050]Embodiments are provided for the detection of cancer by the analysis of a biological sample (e.g., a blood plasma / serum sample) that is not taken directly from a tumor and includes cell-free nucleic acids. The cell-free nucleic acids can originate for various types of tissue throughout the body. In this manner, a broad analysis for the detection of various cancers can be performed.
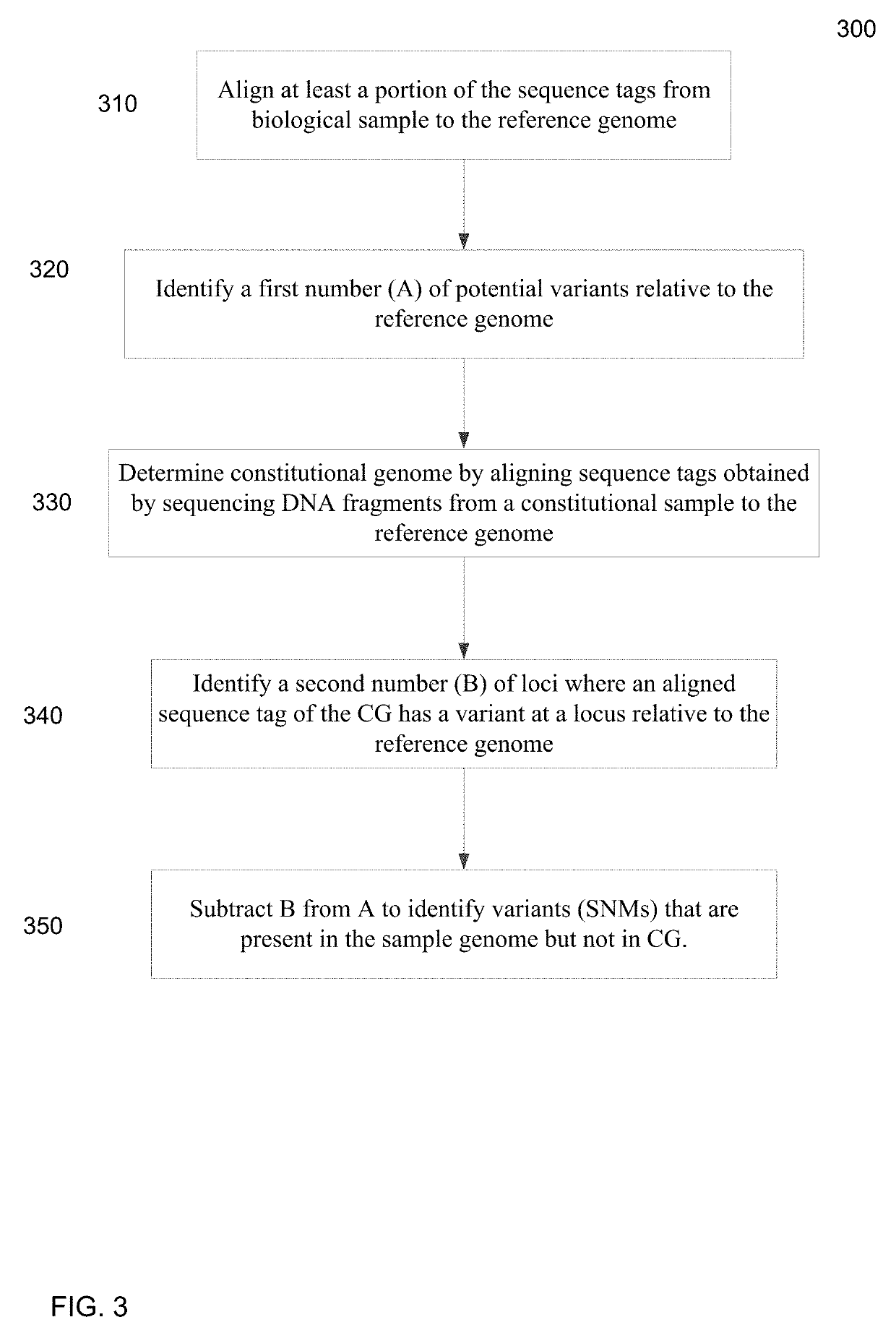
[0051]Genetic aberrations (including single nucleotide mutations, deletions, amplifications, and rearrangements) accumulate in the tumor cells during the development of cancers. In embodiments, massively parallel sequencing can be used to detect and quantify the single nucleotide mutations (SNMs), also called single nucleotide variations (SNVs), in body fluids (e.g. plasma, serum, saliva, ascitic fluid, pleural fluid and cerebrospinal fluid) so as to detect and monitor cancers. A quantification of the number of SNMs (or other types of mutations) can provide a mechanism for identifying early stages of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| threshold | aaaaa | aaaaa |
| tumor heterogeneity | aaaaa | aaaaa |
| absolute concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com