Use of dianhydrogalactitol and analogs or derivatives thereof in combination with platinum-containing antineoplastic agents to treat non-small-cell carcinoma of the lung and brain metastases
a technology of dianhydrogalactitol and an antineoplastic agent, which is applied in the field of hyperproliferative diseases including oncology, can solve the problems of inability to meet preclinical testing and federal regulatory requirements for clinical evaluation, failure or disappointment of compounds that have successfully met preclinical testing and clinical evaluation requirements, and chemical agents where in vitro and in vivo, so as to suppress the growth of cancer stem cells, improve survival, and free of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
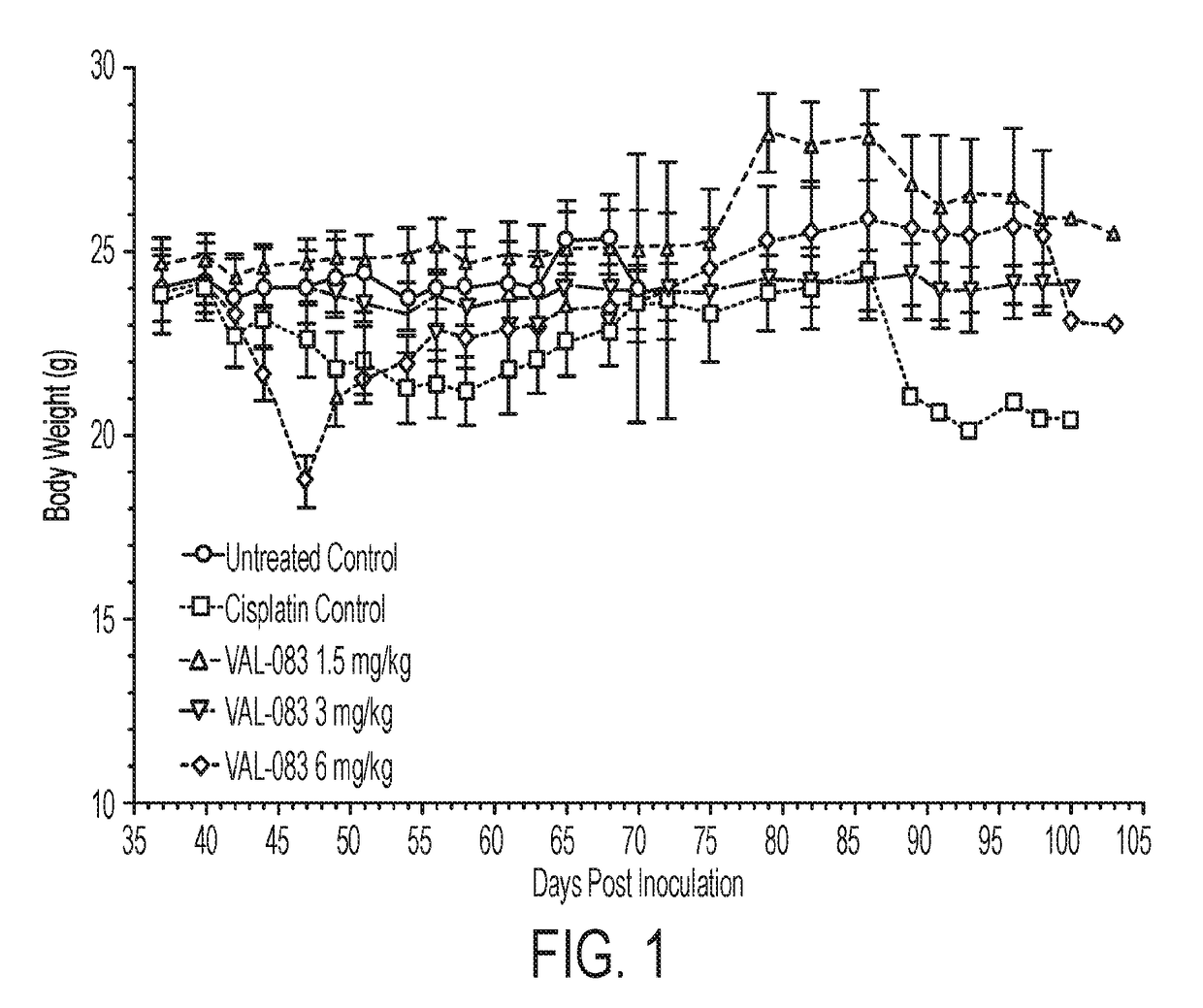
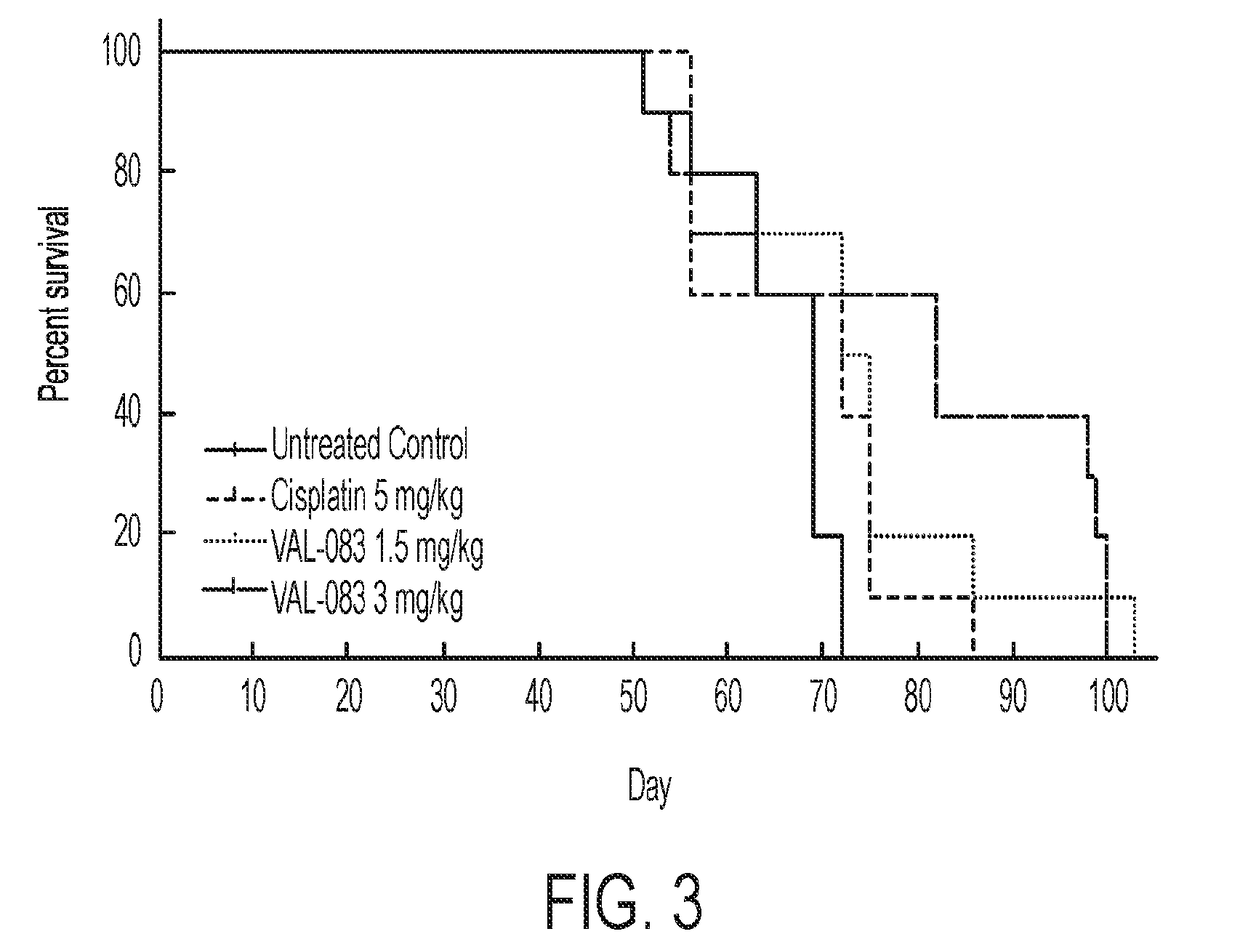
In Vivo Efficacy of Dianhydrogalactitol in the Treatment of Non-Small-Cell Lung Cancer Employing a Mouse Xenograft Model
[1029]Background
[1030]The median overall survival time for patients with stage IV non-small cell lung cancer (NSCLC) is 4 months, and 1- and 5-year survival is less than 16% and 2%, respectively. NSCLC is usually treated with surgery followed by treatment with either Tyrosine Kinase Inhibitors (TKIs) (e.g., erlotinib, gefitinib) or platinum-based regimens (e.g. cisplatin). TKIs have resulted in vastly improved outcomes for patients with EGFR mutations; however, TKI resistance has emerged as a significant unmet medical need, and long-term prognosis with platinum-based therapies is poor. Additionally, the incidence of brain metastases is high in patients with NSCLC with a poor prognosis.
[1031]Dianhydrogalactitol is a structurally unique bi-functional alkylating agent mediating interstrand DNA crosslinks at targeting N7 of guanine, thus differing in mechanism of actio...
example 2
Use of Dianhydrogalactitol as a Novel Treatment Option for Chemo-Resistant Non-Small-Cell Lung Cancer
[1069]The WHO predicts that the incidence of lung cancer may exceed 1 million cases per year by 2025 with non-small cell lung cancer (NSCLC) representing up to 90% of newly diagnosed cases. The median overall survival time for patients with stage IV NSCLC is 4 months, while 1- and 5-year survival is less than 16% and 2%, respectively. Metastatic NSCLC is usually treated with either Tyrosine Kinase Inhibitors (TKIs) (e.g. gefitinib) or platinum-based regimens (e.g. cisplatin). TKIs have resulted in vastly improved outcomes for patients with EGFR mutations; however, TKI resistance has emerged as a significant unmet medical need, and long-term prognosis with platinum-based therapies is poor. Additionally, the incidence of brain metastases is high in patients with NSCLC with a poor prognosis. In particular, NSCLC represents approximately 90% of the lung cancer cases diagnosed in China.
[1...
example 3
Further Results on Cell Lines
[1095]Background
[1096]The median overall survival time for patients with stage IV non-small cell lung cancer (NSCLC) is 4 months, and 1- and 5-year survival is less than 16% and 2%, respectively. NSCLC is usually treated with surgery followed by treatment with either Tyrosine Kinase Inhibitors (TKIs) or platinum-based regimens (e.g. cisplatin). TKIs have resulted in vastly improved outcomes for patients with EGFR mutations; however, TKI resistance has emerged as a significant unmet medical need, and long-term prognosis with platinum-based therapies is poor. Dianhydrogalactitol is a structurally unique bifunctional alkylating agent mediating interstrand DNA crosslinks at N7 of guanine, thus differing in mechanism of action from TKIs and cisplatin. Dianhydrogalactitol has demonstrated activity against NSCLC in preclinical and clinical trials, suggesting dianhydrogalactitol may be a therapeutic option for drug-resistant NSCLC. Dianhydrogalactitol is approve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular mass | aaaaa | aaaaa |
| shelf temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com