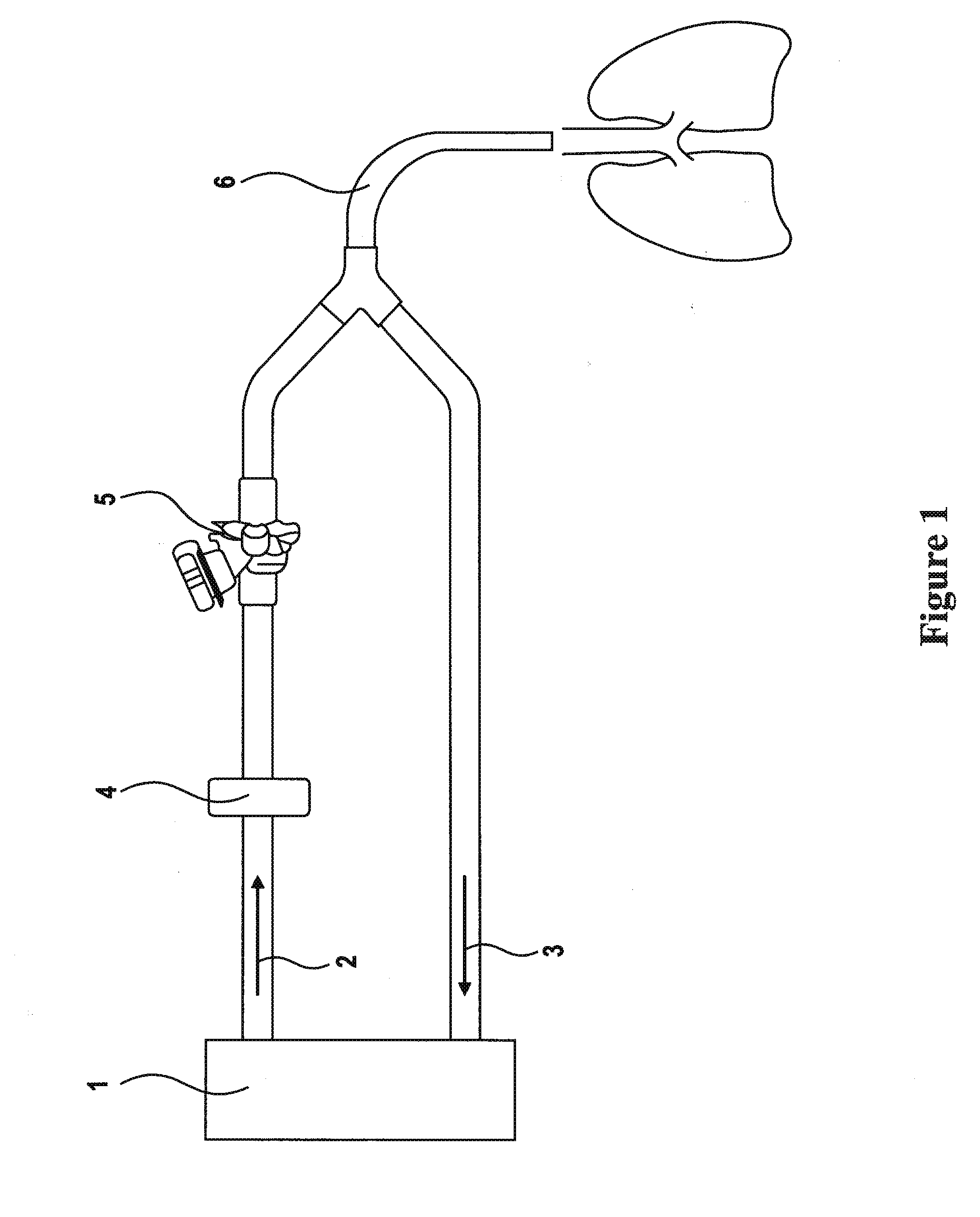
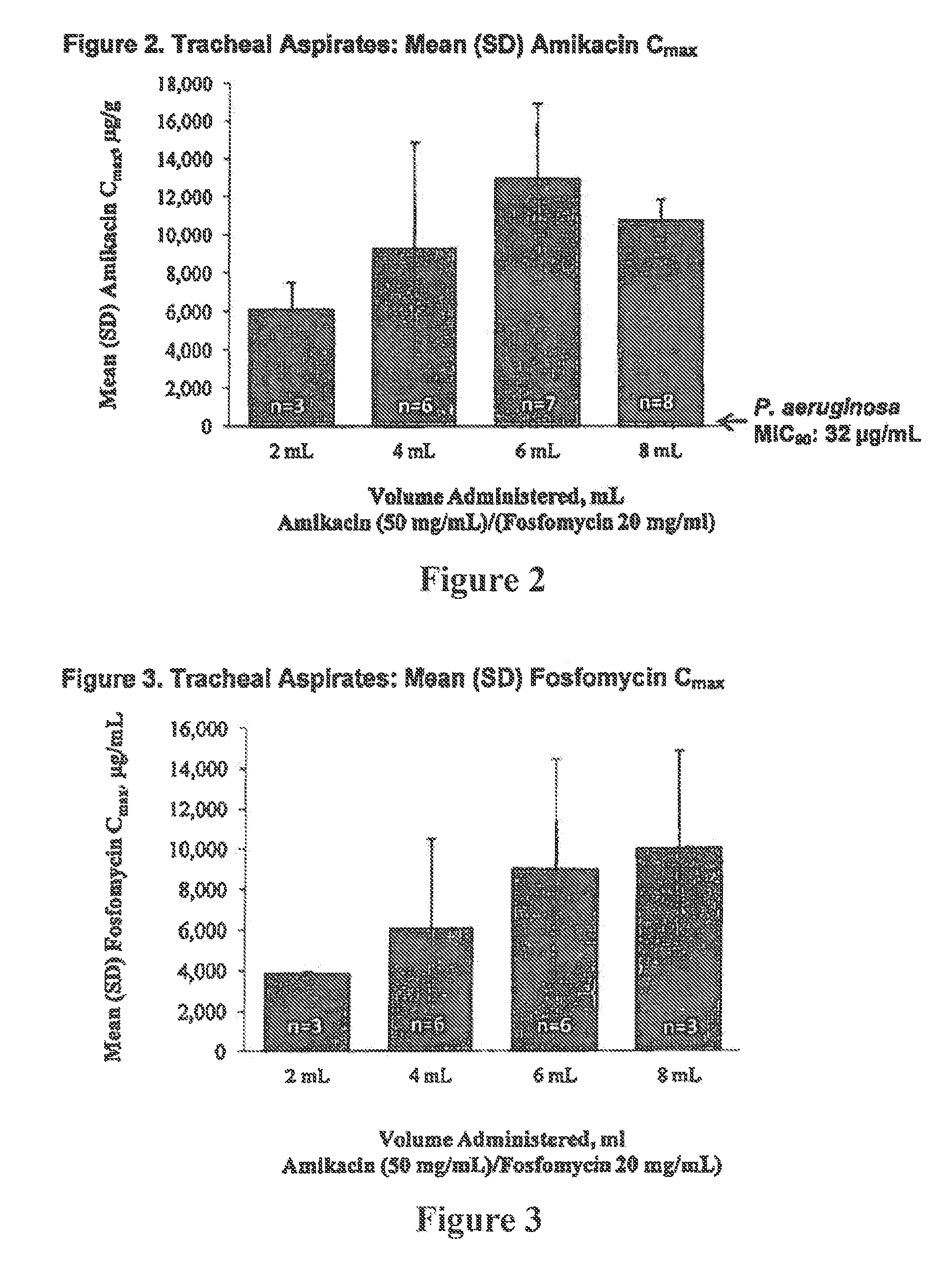
Formulations of aminoglycoside and fosfomycin combinations and methods and systems for treatment of ventilator associated pneumonia (VAP) and ventilator associated tracheal (VAT) bronchitis
a technology which is applied in the field of formulations of aminoglycoside and fosfomycin combinations and methods and systems for treating ventilator-associated pneumonia and ventilator-associated tracheal pneumonia (vat) bronchitis, can solve the problems of poor activity against gram negative bacteria in biofilms, limited activity of mrsa inhibitors, and inability to inhalation therapy. to achieve the effect o
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example # 1
Example #1
Preparation of Fosfomycin / Amikacin Solution for Aerosolization
[0046]A solution having a ratio of amikacin to fosfomycin of equal to or greater than 2.5-2.6:1 is prepared as follows: Fosfomycin disodium (12.90 g, 10.00 g free acid) was dissolved in 250 mL of water and the pH was adjusted to 7.41 by the dropwise addition of 4.5 N HCl (estimated 1 mL). 25 gm Amikacin base was added to the resulting solution. The pH of the solution was adjusted to 7.60 by the addition of 4.5 N HCl (total amount of 4.5 N HCl was 1.7 mL). The solution was diluted to 500 mL with water and filtered through a 0.2 μm Nalge Nunc 167-0020 membrane filter for sterility. The chloride content can be calculated by using 1.7 mL of 4.5N HCl in 50 L total for a total 306 mg chloride. As 1 mEq Cl=35.5 mg in 1 L then in 50 mL 1 mEq Cl=1.775 mg. Therefore, 306 mg / 1.775 mg=172.4 mEq / L. The osmolality of this formulation was measured at 592 mOsm / kg, which is above the normal physiologic value of 310 mOsm.
example # 2
Example #2
Reduction of the Osmolality of the Solution by Humidification
[0047]A solution having a fosfomycin / amikacin ratio of 2.5-2.6-1 amikacin to fosfomycin was prepared as above. Using an inline electronic vibrating late nebulizer (PARI, Starnberg GR), the formulation was nebulized in dry (4%) and humid (100%) humidity. The mean particle size, as measured by Malvern X laser particle sizer, was 2.9 μm under dry conditions, increasing to 3.2 μm under 100% humidity.
[0048]Since the volume of sphere is function of the third power of the radius, the following equation yields the dilution factor:
1.45×1.45×1.451.6×1.6×1.6=0.75
[0049]Thus, the formulation on average is diluted by a factor of 0.75, indicating the delivered formulation has an osmolality of 592×0.75=444 mOsm / Kg.
example # 3
Example #3
Randomized, Double-Blind, Placebo-controlled, Dose-escalation Phase 1b Study of Aerosolized Amikacin and Fosfomycin Delivered Via the PARI Investigational eFlow® Inline Nebulizer System in Mechanically Ventilated Patients
[0050]A dry powder fosfomycin, liquid amikacin solution can be prepared by use of 200 mg neat dry powder disodium fosfomycin filled in a glass vial or two-part dry liquid syringe. In either a separate syringe, blow fill seal container, or a two-part syringe, 500 mg of amikacin base dissolved in 10 mL of sterile water, with the pH adjusted to a range of 4.5 to 7.5 with HCl. The two components are then mixed together giving a solution with 20 mg / mL fosfomycin, 50 mg / ML amikacin. The osmolality of the solution would be approximately 600 mOsm / Kg, but could vary up to 10% depending on the amount of HCl used to adjust the pH of the amikacin solution. Also by employing the chlorine counter anion with the amikacin base, the sulfate salt of amikacin is not used.
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com