Relaxin for treating patients afflicted of impaired glucose tolerance
a glucose tolerance and relaxin technology, applied in the field of relaxin for treating patients with impaired glucose tolerance, can solve the problem of not being able to specify the exact “effective amount” or “effective dose”, and achieve the effect of increasing patient compliance and therapeutic effect, preventing beta-cell loss, and reducing the rate of relaxin releas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
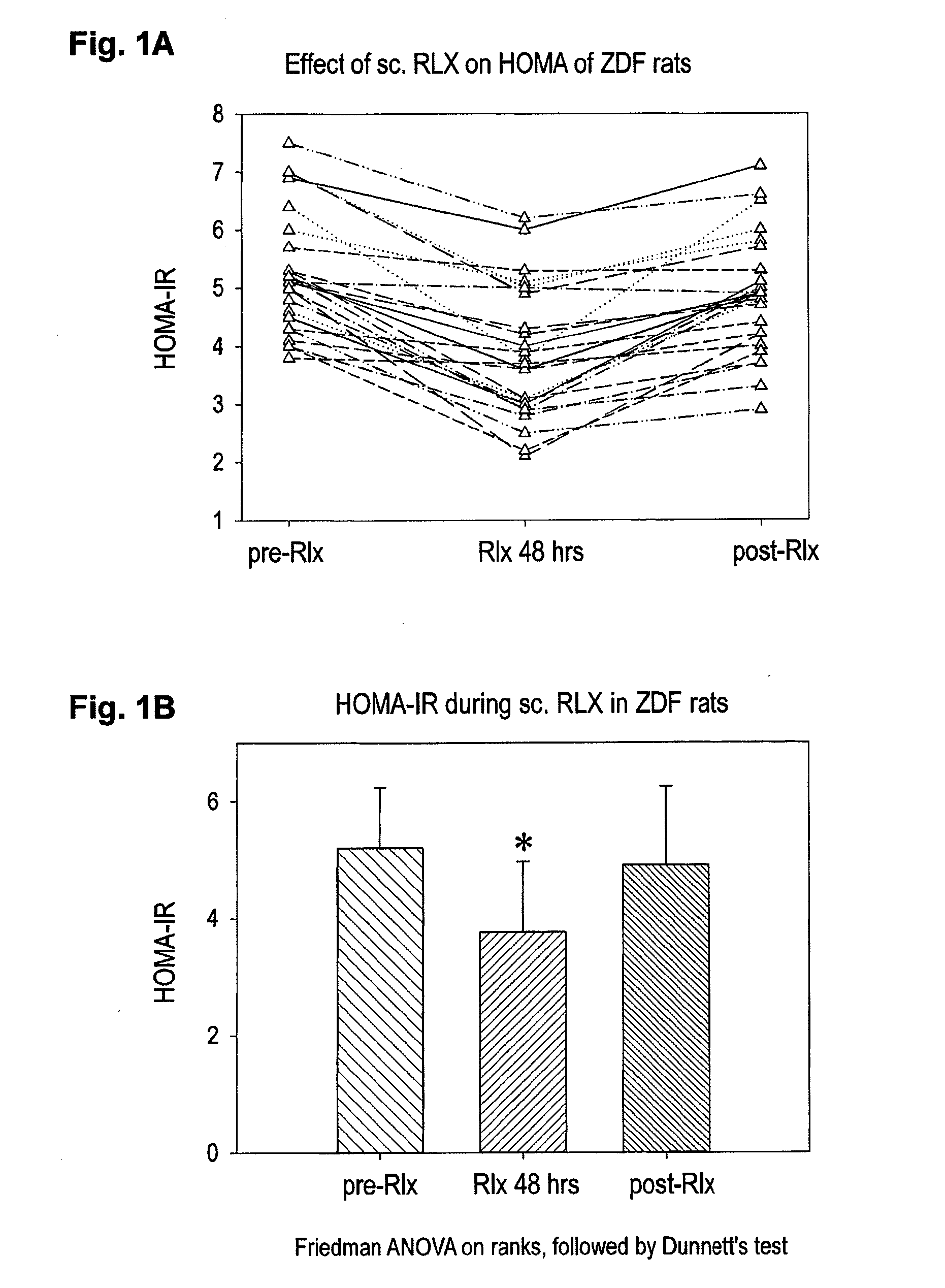
Effect of sc RIX on HOMA-Index in ZDF Rats
[0038]The homeostatic model assessment (HOMA=Insulin (μU / mi)×Glucose (mmol / l) / 22,5) allows a quantification of insulin resistance and beta-cell function on the basis of mathematical equations describing glucose regulation as a feedback loop (see Matthews DR at al. Diabetologia 1985; 28(7): 412-9); Turner RC et al, Metabolism 1985; 28 (11): 1086-96). In order to assess the effects of relaxin on a diabetic metabolism we determined the HOMA of 10-week old male Zucker Diabetic Fatty rats (#25) pre-, during and post subcutanous administration of porcine relaxin (500 μg / kg rat per day). Thus, we determined the glucose and insulin levels in serum of 25 ZDF rats prior and 48 hours after sc administration of porcine relaxin. This was completed by follow-up determinations of glucose and insulin concentrations in serum 48 hours post the termination of the administration of porcine relaxin. The respective results for the HOMA values are shown in FIGS. 1...
example 2
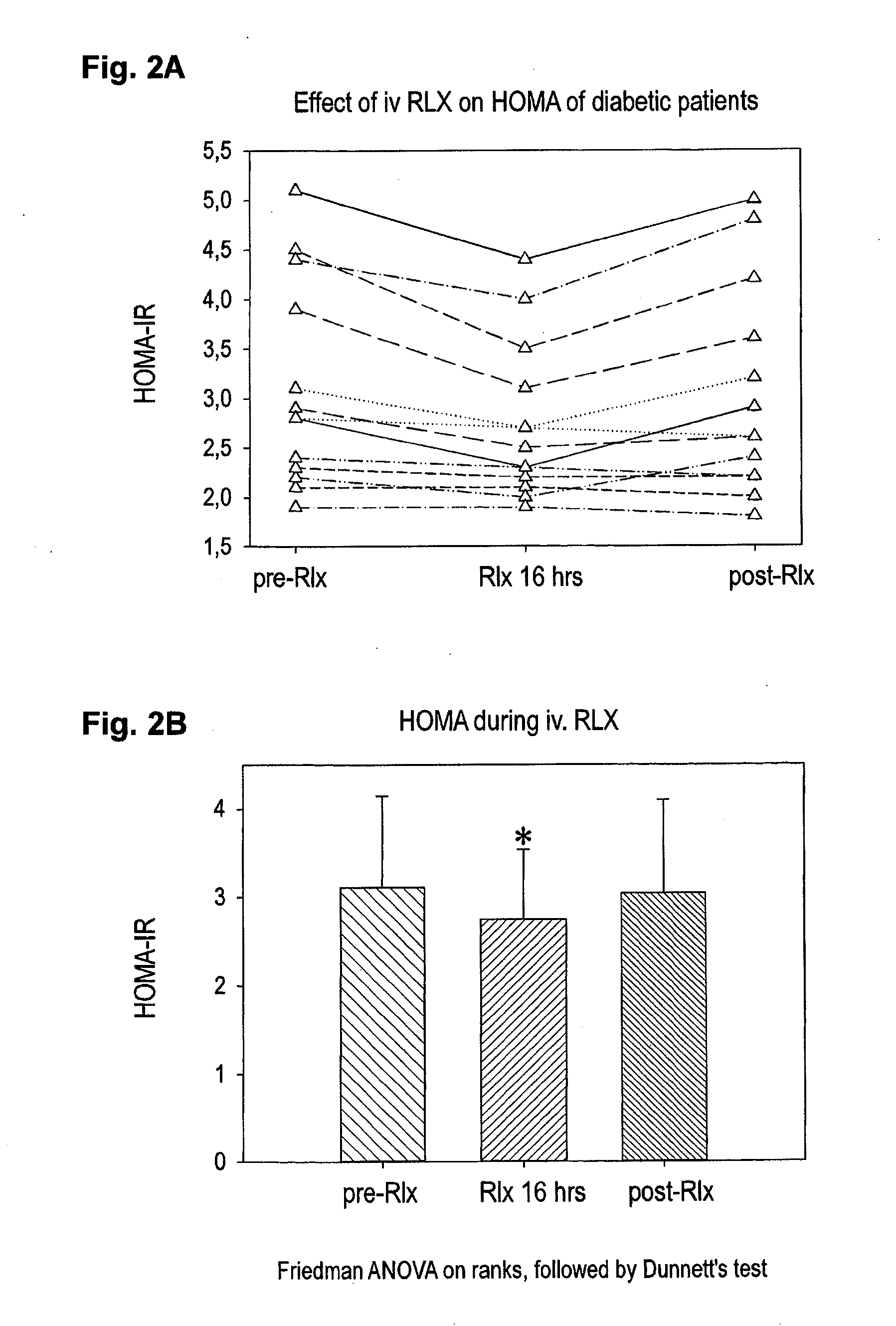
Effect of H2 RLX on HOMA-Index in Human Patients
[0040]In a study approved by the local ethical committee (Charité, Berlin, Germany) twelve patients (n=12) having type II diabetes or impaired glucose tolerance received intravenous synthetic H2 relaxin over a period of 16 hrs followed by a 24-hr follow-up . The anti-diabetic treatment of those patients was not varied. The improved HOMA-2 model used was in the present example because it reflects better human physiology. A HOMA of >2 indicates a potential insulin resistance; a HOMA of >2,5 beginning insulin resistance and a HOMA >5,0 is an average value of insulin resistance in untreated patients with manifest type-2 diabetes.
[0041]Of those twelve patients in this study two patients received sequential treatment for 8 hours each with dosages equivalent to 10 and 30μg / kg of subject body weight per day, 6 patients received sequential treatment with 240 and 480 μg / kg of subject body weight per day, and another 4 patients received a constan...
example 3
Influence of Sustained Glucose Concentration on the Function of Beta-Cells and Their Secretion of Insulin into the Supematant
[0043]In a further study we examined whether porcine relaxin has any effect on insulin expression and / or secretory capacity of pancreatic beta-cells. 2.5×105 rat insulinoma cells (INS-1 cells; see Mergler S et al, in Cellular Signalling (2008), doi: 10.1016 / j.cellsig.2008.08.015) were plated in a 24-well plate and grown for three days in RPMI-640 medium with supplements (Moore G. E. et. al., Culture of Normal Human Leukoctyes.” JAMA, v. 199, 519-524 (1967). On the day of the experiment, the medium was removed, and the INS-1 cells were washed three times and then preincubated for 30 min in KRB (Krebs-Ringer buffer: 115 mM NaCl, 5.9 mM KCl, 1.2 mM MgCl2, 1.2 mM NaH2PO4, 1.2 mM Na2PO4, 2.5 mM CaCl2, 25 mM NaHCO3, pH 7.4) comprising 0.5% BSA and 3.3 mM glucose. Cells were washed again with phosphate buffered saline (PBS: 137 mM NaCl, 2,7 mM KCl, 12 mM phosphate, p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com