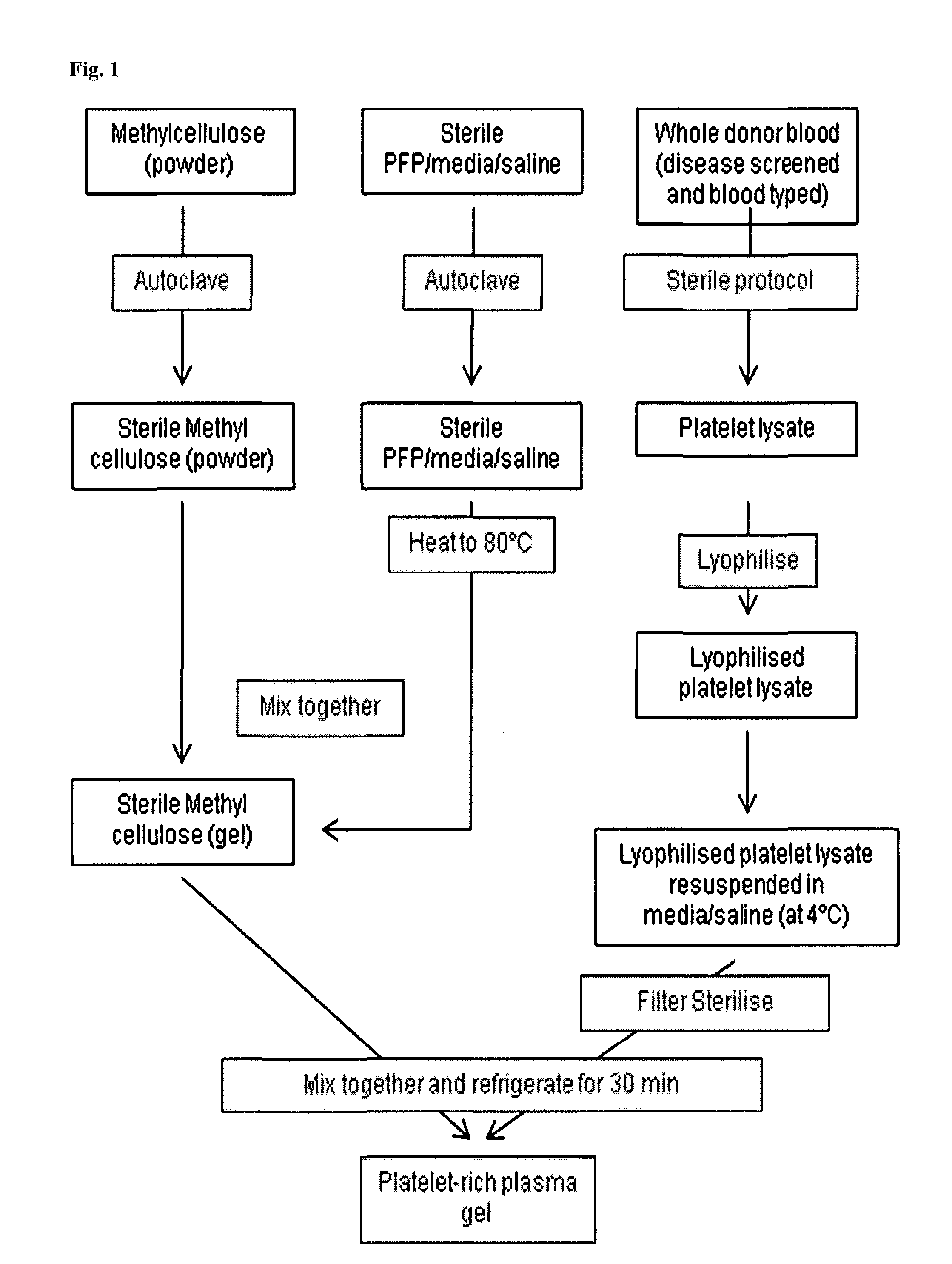
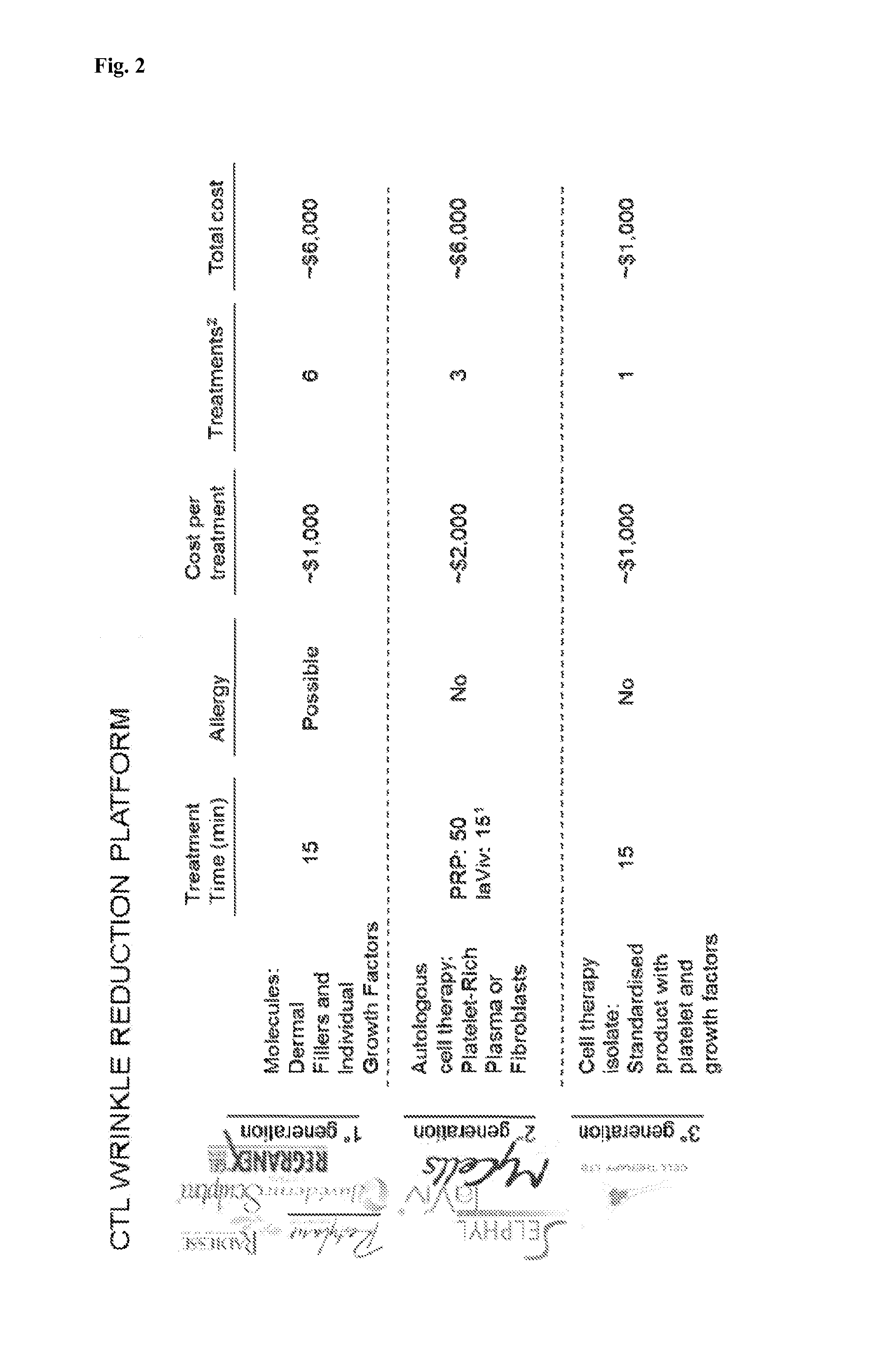
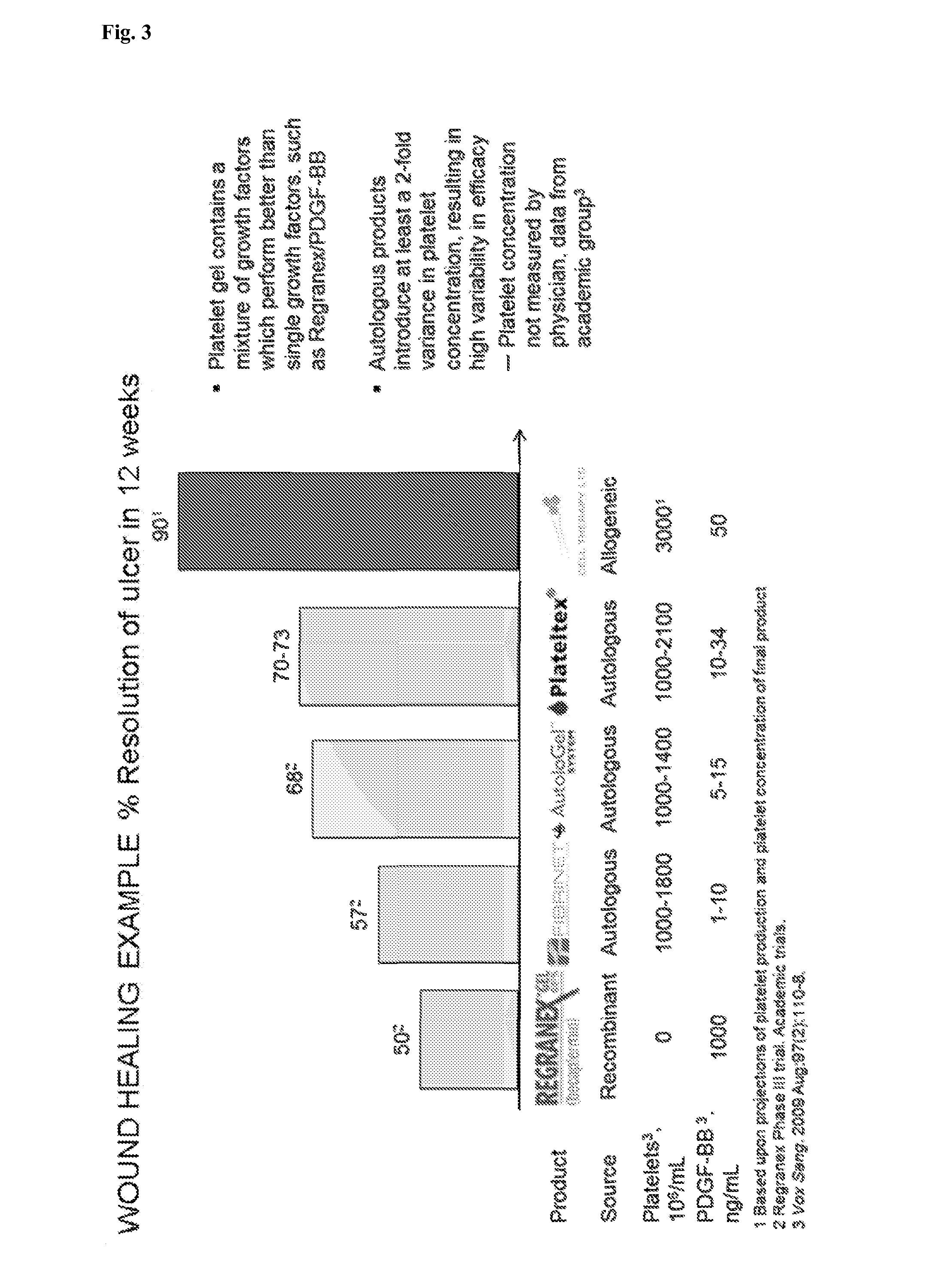
Platelet lysate gel
a technology of platelet lysate and gel, which is applied in the direction of drug compositions, bandages, sexual disorders, etc., can solve the problems of limited technological breakthroughs in wound healing, limited improvement of advanced dressings such as hydrocolloid gel over traditional non-adherent gauze, and inability to irritate the skin patch, etc., to achieve convenient adaptation for chronic wound healing markets, high yield of safe, and simplified current process for physicians
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Platelet Lysate
[0138]A sample of whole blood was collected and saved for analysis of the number of platelets, as a way to monitor the lysis process. The whole blood was centrifuged (120×g, 15 minutes, no break, room temperature) to separate the platelets from the remaining blood cells. The platelets ended up in the yellow plasma, known as platelet rich plasma (PRP), which was resting on top of a dark red pillar of the remaining blood cells.
[0139]A small sample of the PRP was saved to analyze the platelets, and it was show that the platelet concentration is higher compared to the whole blood as the platelets have now been concentrated in a smaller volume. The volume of the plasma differs between individuals but is related to gender such that the volume will be smaller in men and the platelet concentration in whole blood compared to PRP will therefore have increased more in men.
[0140]The PRP was transferred to another container, such as a 50 ml tube, and was then submerged in liquid n...
example 2
Platelet Lysate Gel
[0141]Platelet lysate prepared as in Example 1 was transferred into a graduated sterile Falcon tube. If previously frozen, the platelet lysate was first thawed at room temperature. To the platelet lysate, calcium gluconate and plasma or thrombin were added in appropriate proportions. If the plasma was previously frozen, it was thawed at 37° C. prior to use. Appropriate proportions as were used herein include: e.g. 1:5 parts of platelet lysate, 2 parts of plasma, 2 parts of calcium gluconate; and e.g. 2:3 parts of platelet lysate, 1 part of thrombin, and 0.5 parts of calcium gluconate. The resulting suspension was exposed to careful slow shaking to complete 10-12× 360° tube revolutions and then fractionated by size into sterile dispensation devices. Devices included syringes and micro-needle dispensers. In some instances fractionation by size was determined according to the size and shape of the ulcer to be treated.
example 3
Platelet Lysate Gel Utility
[0142]For treatment of vaginal atrophy: 2-10 ml of plasma lysate gel with viscosity of ˜1000 cps was applied intra-vaginally daily or every other day for 1-2 weeks. This was repeated for a week if symptoms recurred.
[0143]For treatment of a 5 mm diameter Diabetic foot ulcer: 2-3 ml of plasma lysate gel with viscosity of 70,000-100,000 cps was applied topically onto the ulcer daily every other day for 2-3 weeks.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com