Antibody derivatives
a technology of antibodies and derivatives, applied in the field of antibodies derivatives, can solve the problems of marked reduction of production yield of these disulfide, hampered wide-spread development of bispecific antibodies, and difficulty in producing materials of sufficient quality and quantity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of IL6 and IL23 Expression Clones and Purification of Fusion Proteins
[0404]Human IL-6 (DQ891463; ABM82389.1, SEQ ID NOs. 343 and 344) was expressed as a 3×FLAG-IL6-Avi fusion. The expression construct was generated by a two-step polymerase chain reaction (PCR) amplification. The product was inserted into the plasmid p3xFLAG-CMV-23 (Sigma), downstream of the CMV promoter and in frame with the pre-pro-trypsin leader sequence and triple FLAG tag. The expressed IL-6 (SEQ ID NO. 1) contained an amino terminal triple FLAG tag (amino acid sequence dhdgdykdhdidykdddd; SEQ ID NO. 345) and a carboxyl-terminal Avi tag (glndifeaqkiewhe; SEQ IS NO. 346) (ALLO66-MM4-80) The IL6 coding region was amplified by PCR and inserted into p3xFLAG-CMV-23 to express 3×FLAG-IL6 and 3×FLAG-IL6-myc fusions. (SEQ ID NO. 2)
[0405]The coding region of the macaca mulatta IL-6 (mmIL-6; NM—001042733.1; NP—001036198; SEQ ID NO. 346 and SEQ ID NO. 347) was generated by overlapping oligomers and PCR (DNA2.0...
example 2
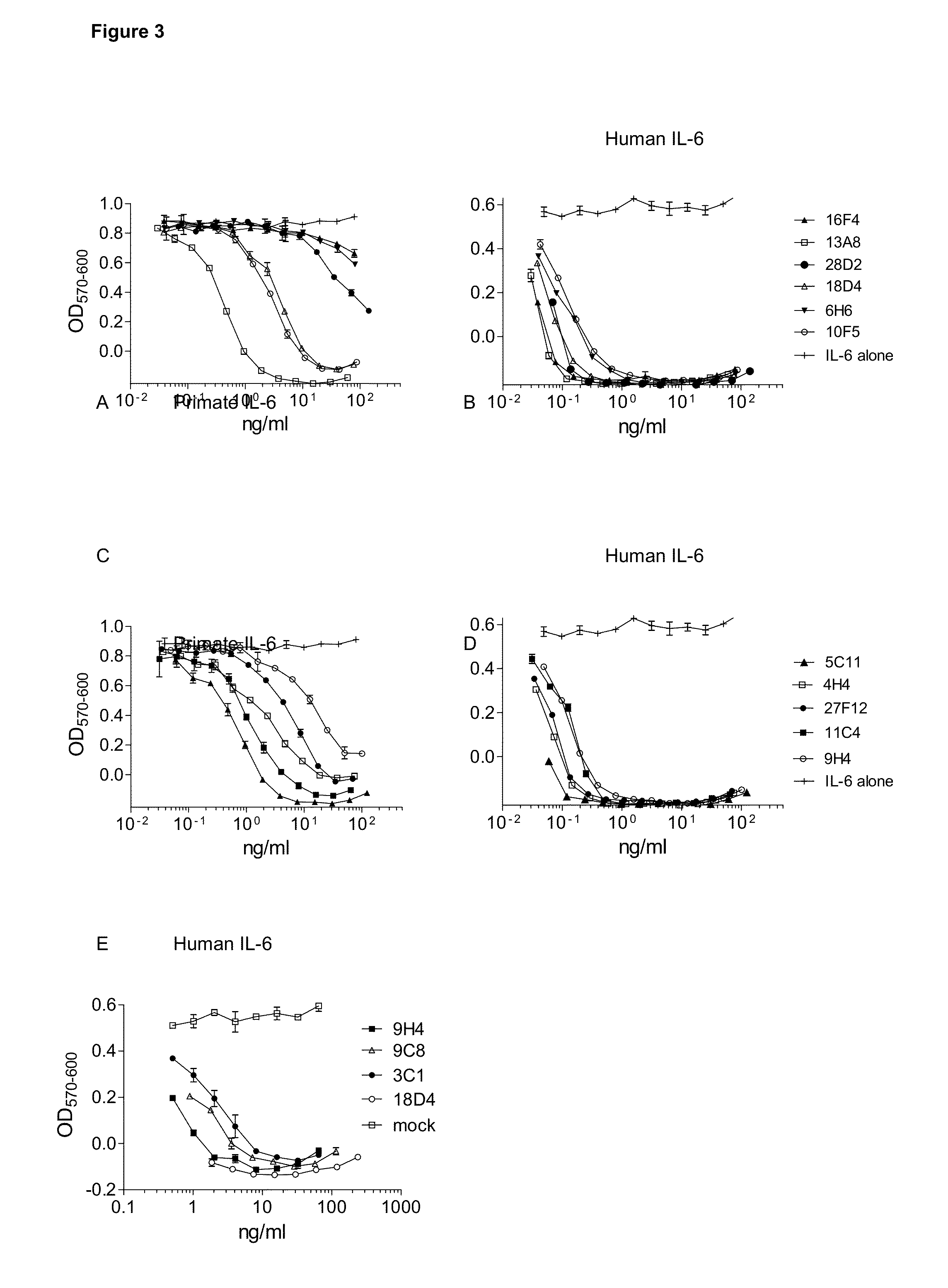
Determination of Antibody Affinity
[0411]Equilibrium dissociation constants were determined by surface Plasmon resonance using a SensiQ Pioneer (ICx Nomadics, Stillwater, Okla.) and a carboxylated COOH1 sensor (Ibid) amenable for amine coupling. Protein G (6510-10, Biovision, Mountain View, Calif.) was coupled to the COOH1 sensor using amine coupling reagents (Sigma Aldrich (N-Hydroxysuccinimide (NHS, 56480), N-(3-Dimethylaminopropyl)-B′-ethylcarbodiimide hydrochloride (EDC, E7750), Ethanolamine (398136), St. Louis, Mo.) or with the Biacore Amine Coupling Kit (BR-1000-50, GE Healthcare, Waukesha, Wis.).
[0412]Briefly, the carboxylated surface is activated with 2 mM EDC and 0.5 mM NHS for a contact time ranging between 2-10 minutes. Protein G, in variable concentrations ranging between 20-400 ug / mL, was diluted into 10 mM acetate buffer, pH 4.3 (sodium acetate, BP334-1; glacial acetic acid, A490-212; Thermo Fisher Scientific, Waltham, Mass.), and injected over the activated sensor for ...
example 3
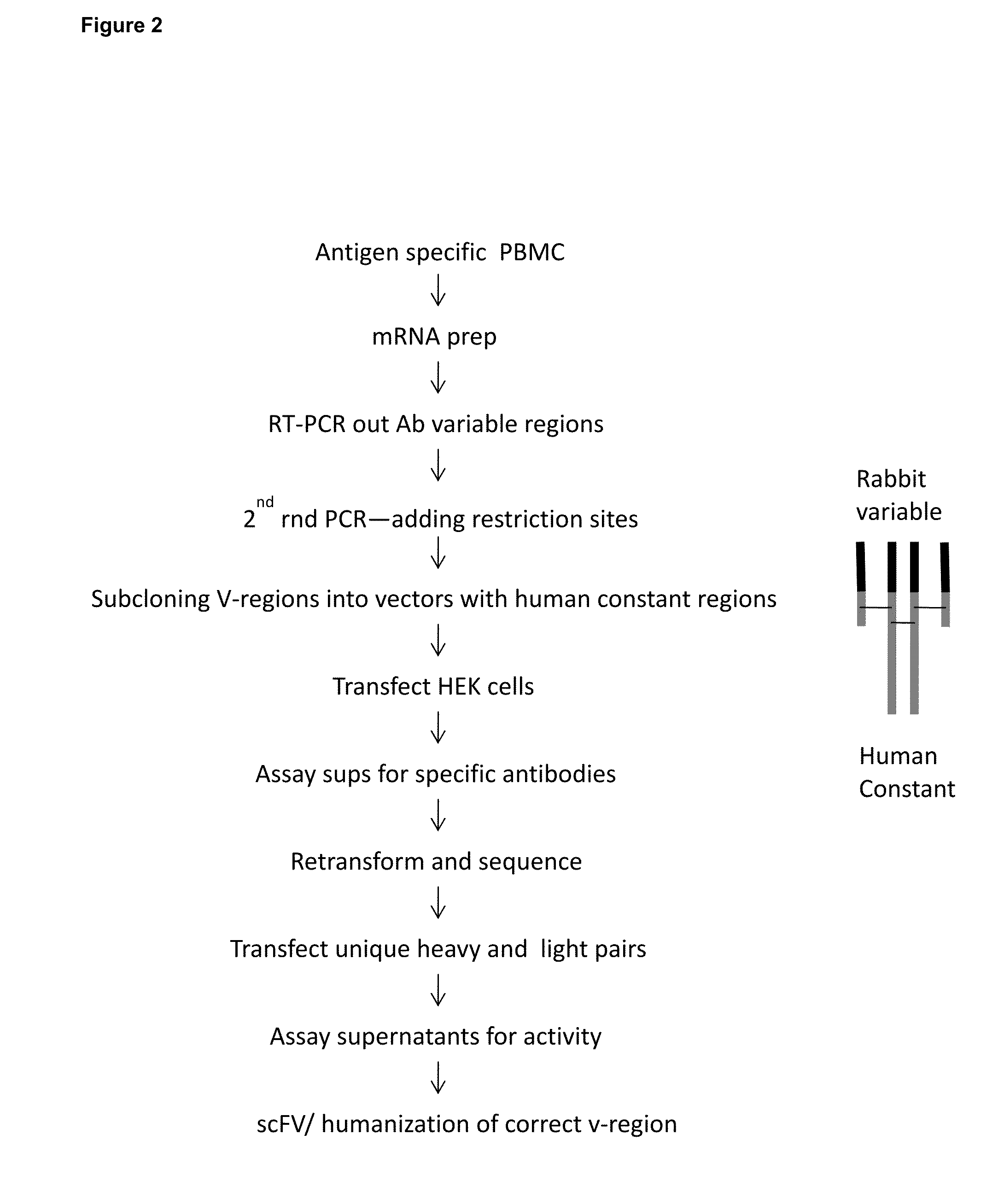
Generation of Rabbit Anti Human IL-6 Monoclonal Antibodies
3.1 Rabbit Immunization:
[0416]One New Zealand White rabbit was immunized with 100 μg of IL-6 protein (Recombinant E. Coli-derived human IL-6, Ref. Seq. accession NP000591.1, obtained from ProSpec-Tany TechnoGene Ltd., Rehovot, Israel (Cat. # CYT-213i)) in Sigma Adjuvant System (Sigma S6322), at days 0, 21, and 42. The rabbit was boosted not less than 10 days prior to bleeding. Rabbits were maintained at R & R Research Laboratories (Stanwood, Wash., USA) in accordance with NIH, USDA and IACUC guidelines.
[0417]30 ml of blood were harvested from each rabbit by venipuncture. Peripheral Blood Mononuclear cells (PBMC) were prepared by density centrifugation (Lympholyte-rabbit, Cat. # CL5050, Cedarlane Laboratories Ltd., Ontario, Canada).
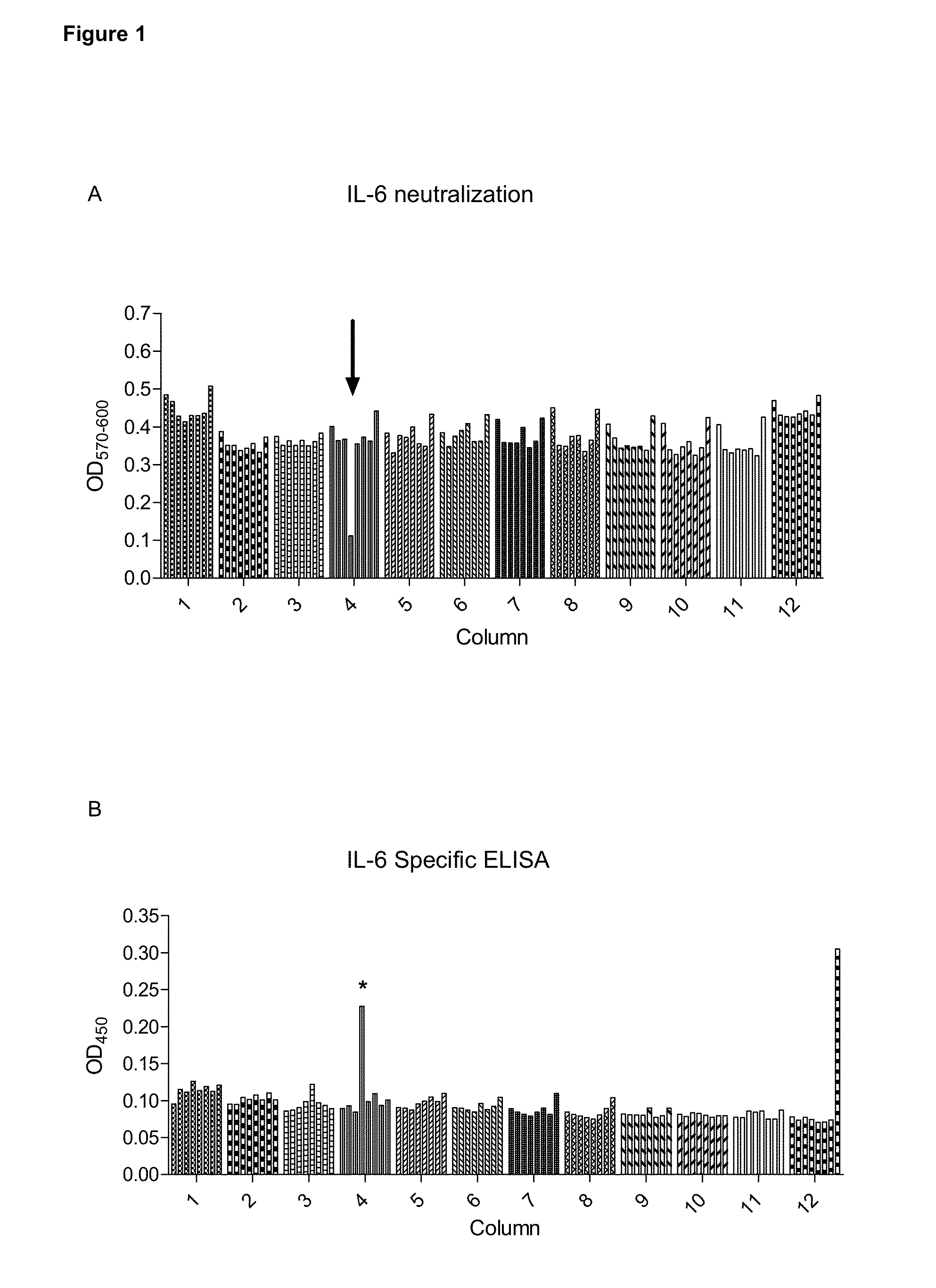
[0418]The neutralizing activity of immune rabbit serum against human IL-6 was assayed after 3 immunizations.
[0419]Human IL-6 is titered from 1 ng / ml with and without a 1:3200 dilu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Water solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com