Regioselectively substituted cellulose esters and efficient methods of preparing them
a technology of cellulose esters and regioselective substitution, applied in the field of chemistry, can solve the problems of difficult to synthesize regioselectively substituted cellulose esters, difficult to achieve general solutions, and difficult to achieve high regioselectivity in cellulose ester syntheses, and achieve the effect of efficient production of desired materials and simplified methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0055]Reference will now be made in detail to various exemplary embodiments of the invention. It is to be understood that the following discussion of exemplary embodiments is not intended as a limitation on the invention. Rather, the following discussion is provided to give the reader a more detailed understanding of certain aspects and features of the invention.
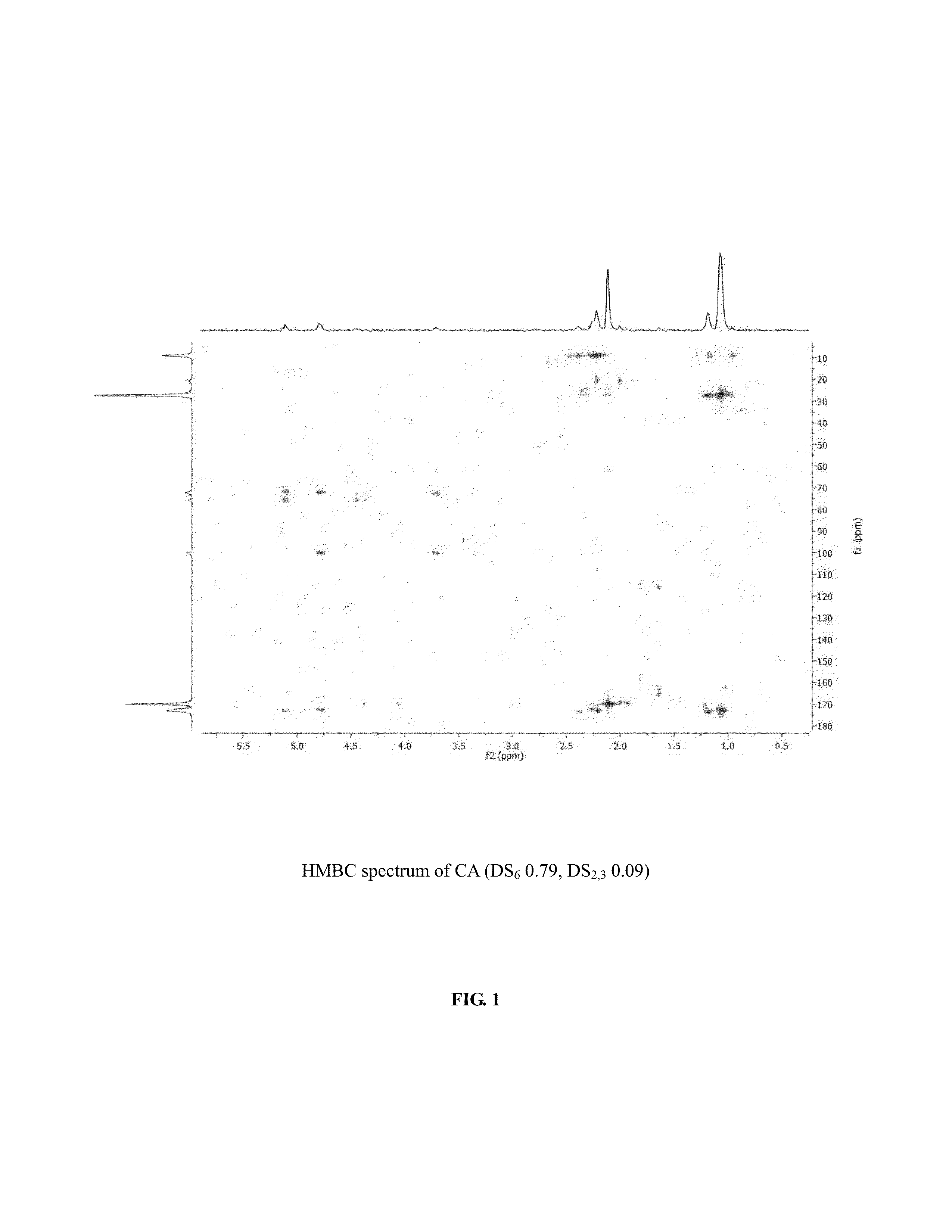
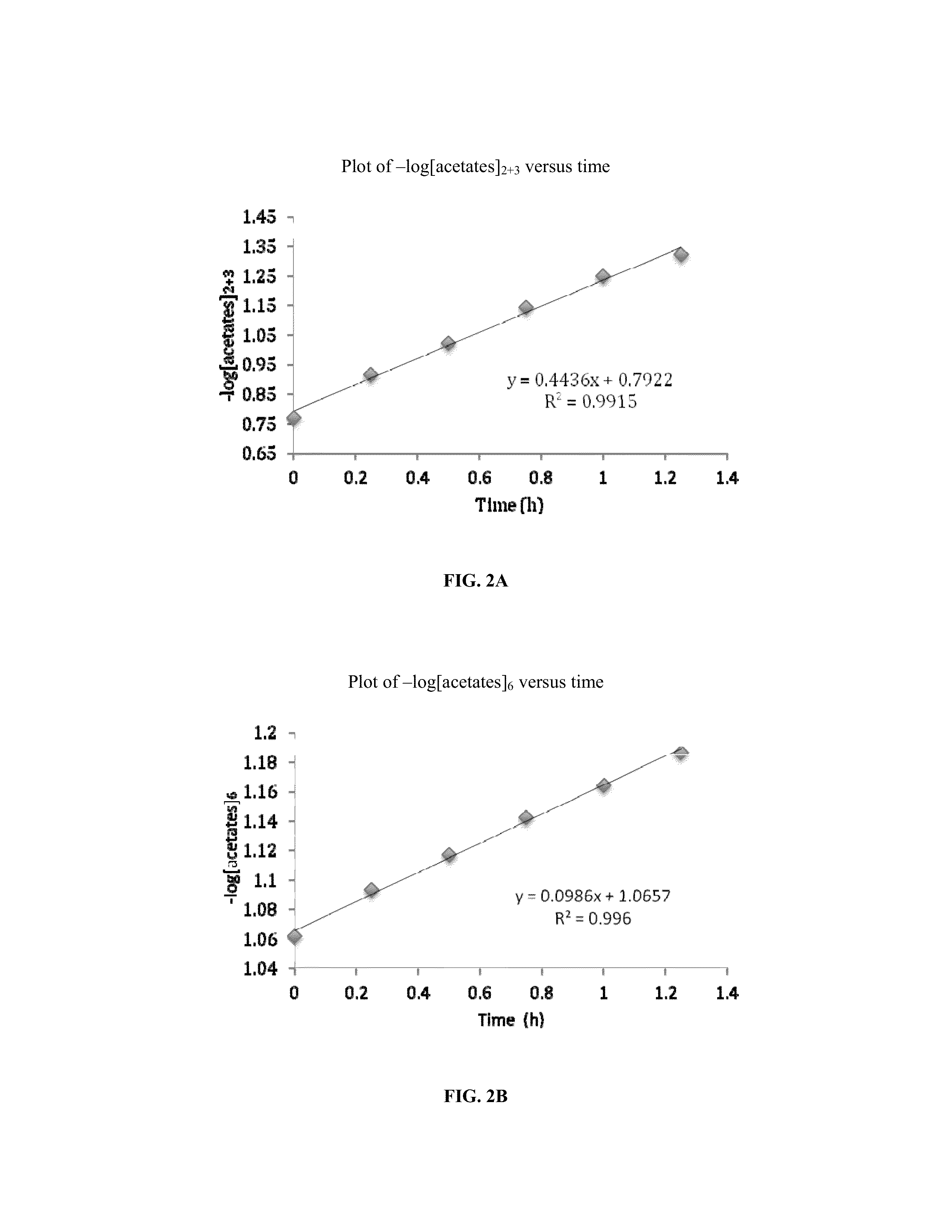
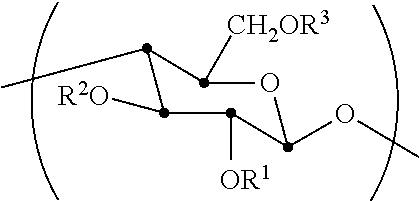
[0056]The synthesis of cellulose-2,6-A-3-B—O triesters (in other words, esters of cellulose in which one ester type is attached at O-3 (type A, for example acetate), and a second type is attached at the O-2 and O-6 positions (type B, for example propionate)) with a high degree of regioselectivity has been demonstrated using protection of cellulose at both 2- and 6-OH groups using bulky silyl ethers. See Xu, D., Voiges, K., Elder, T., Mischnick, P., Edgar, K. J. Biomacromolecules 2012, 13, 2195. While quite valuable for determining the regiochemical structure-property relationships, such methods are limited in scope. One disa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Nanoscale particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com