Pharmaceutical composition comprising a curcumin derivative
a technology of curcumin and composition, applied in the field of pharmaceutical composition comprising a curcumin derivative, can solve the problems of high cost, high cost, and high cost, and achieve the effects of improving curcumin brain bioavailability, reducing the dose required, and high lipophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
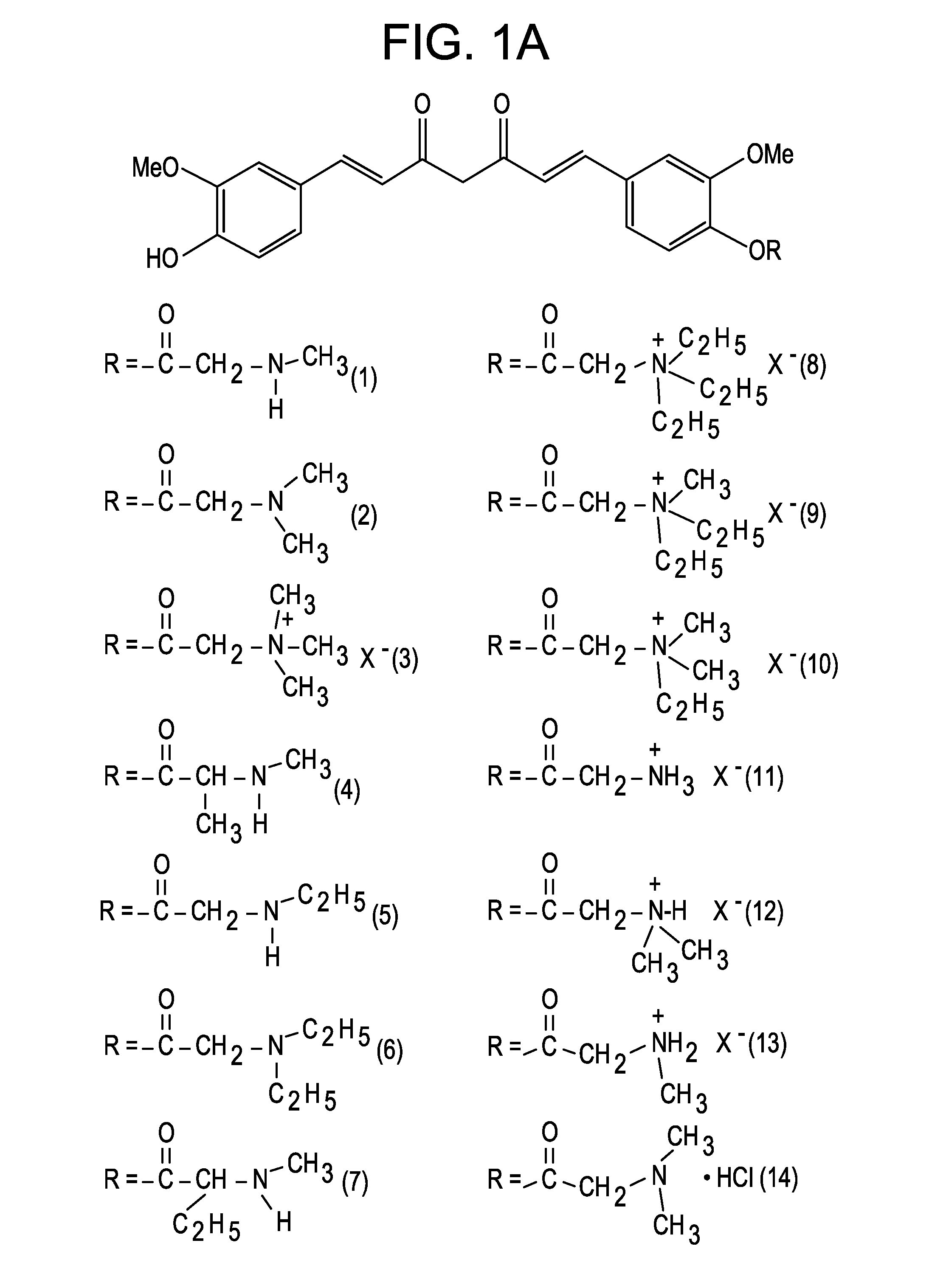
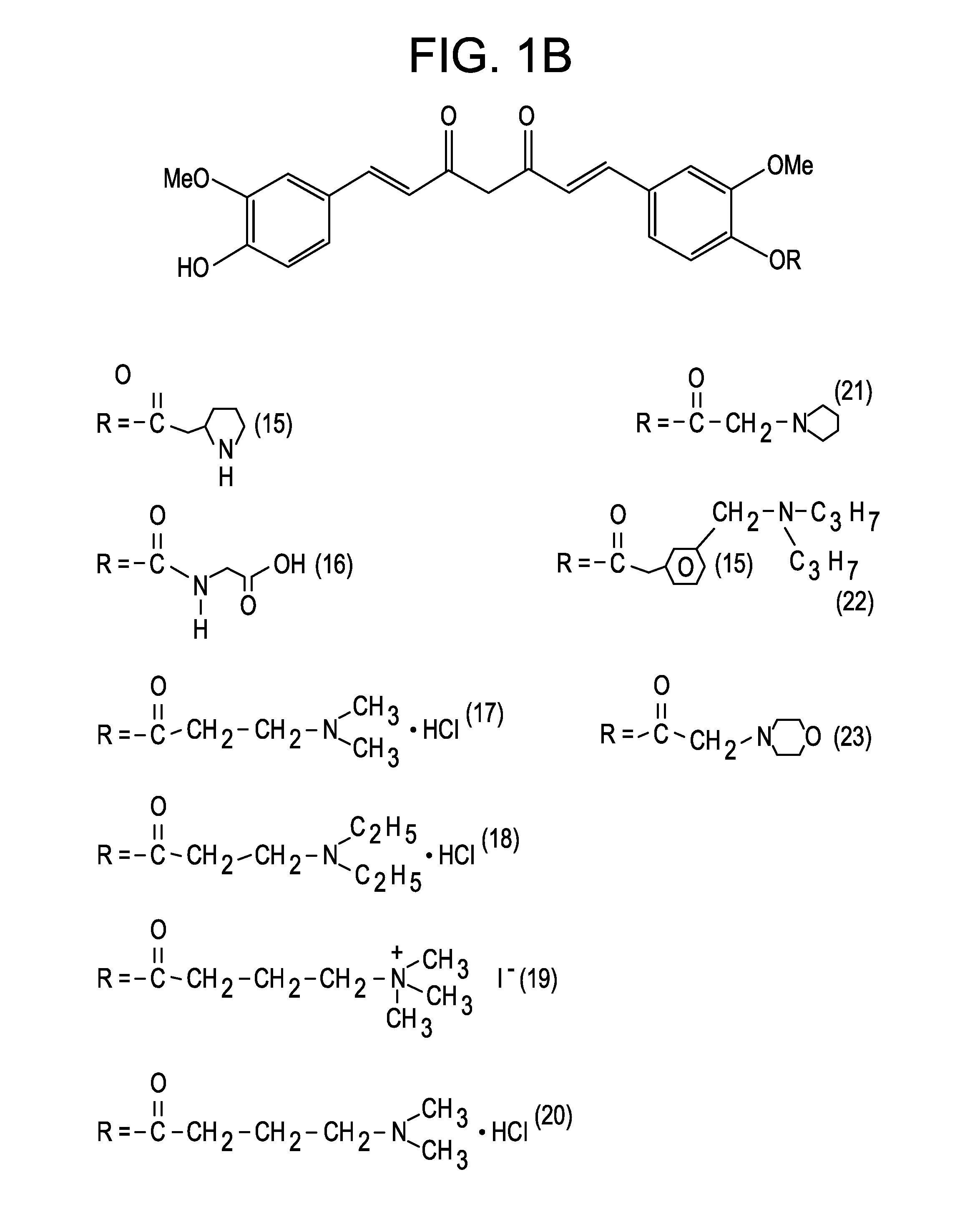
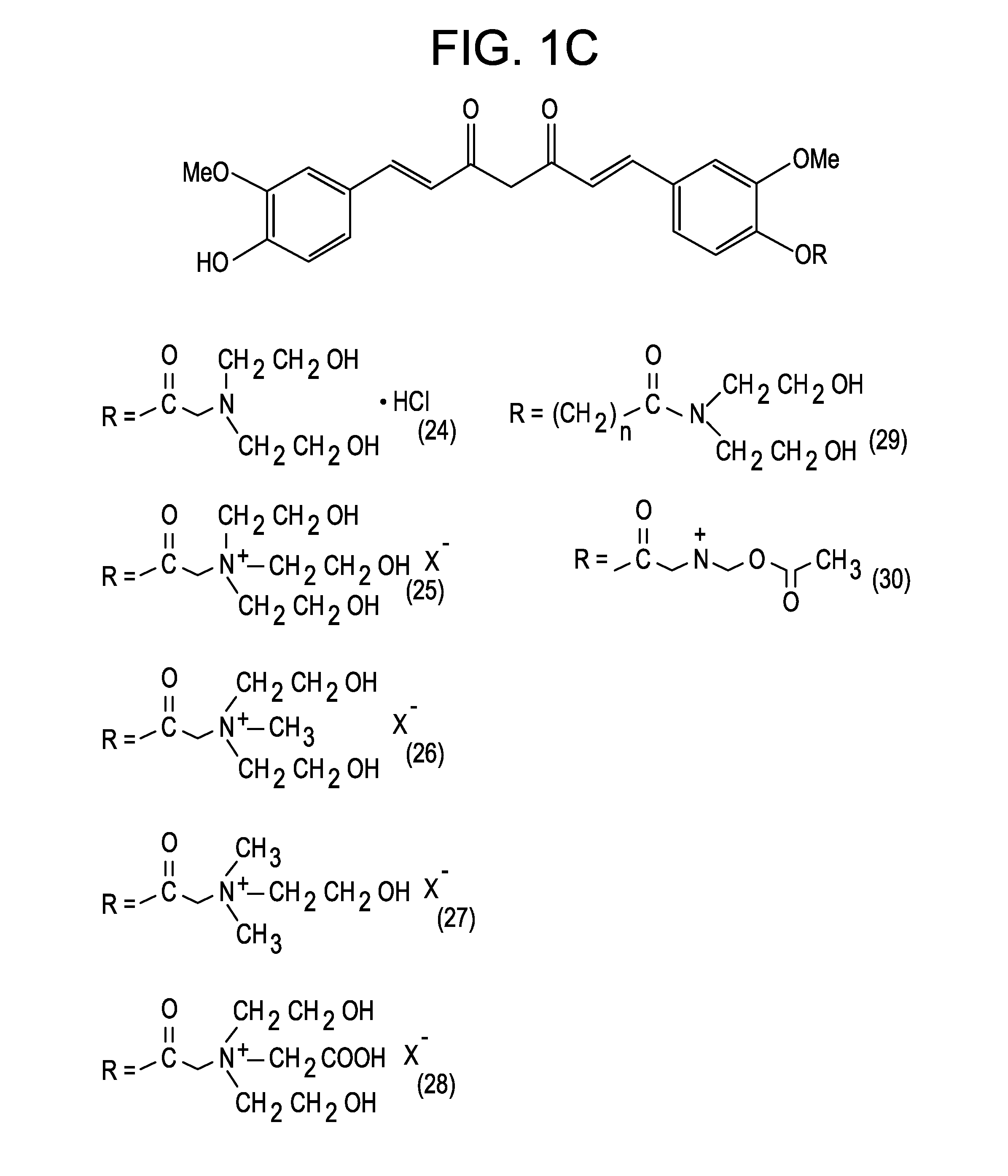
[0047]The present invention is directed to a compound represented by Structural Formula (I) or a pharmaceutically acceptable salt thereof. Values and alternative values for the variables in Structural Formula (I) and for each of the embodiments described herein are defined as the following: represents a single bond or a double bond. In one embodiment, represents a double bond.
[0048]Each R1, R2 and R3 are independently selected from the group consisting of —OH, —O(C1-C6)alkyl, halo, —C(Y)3 and —OP. In one embodiment, each R1, R2 and R3 are independently selected from the group consisting of —OH, —O(C1-C6)alkyl and —OP. In another embodiment, each R1, R2 and R3 are independently selected from the group consisting of —OH, —OCH3 and —OP. In a further embodiment, each R1, R2 and R3 are independently —OH or —OCH3.
[0049]Y is a halogen (—F, —Cl, —Br or —I). In one embodiment, Y is —F, —Cl or —Br.
[0050]P is a hydrolyzable group. In one embodiment, P is selected from the group consisting of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com