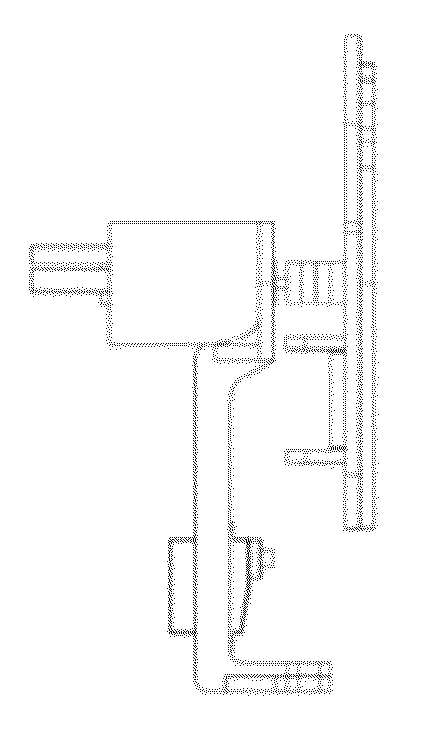
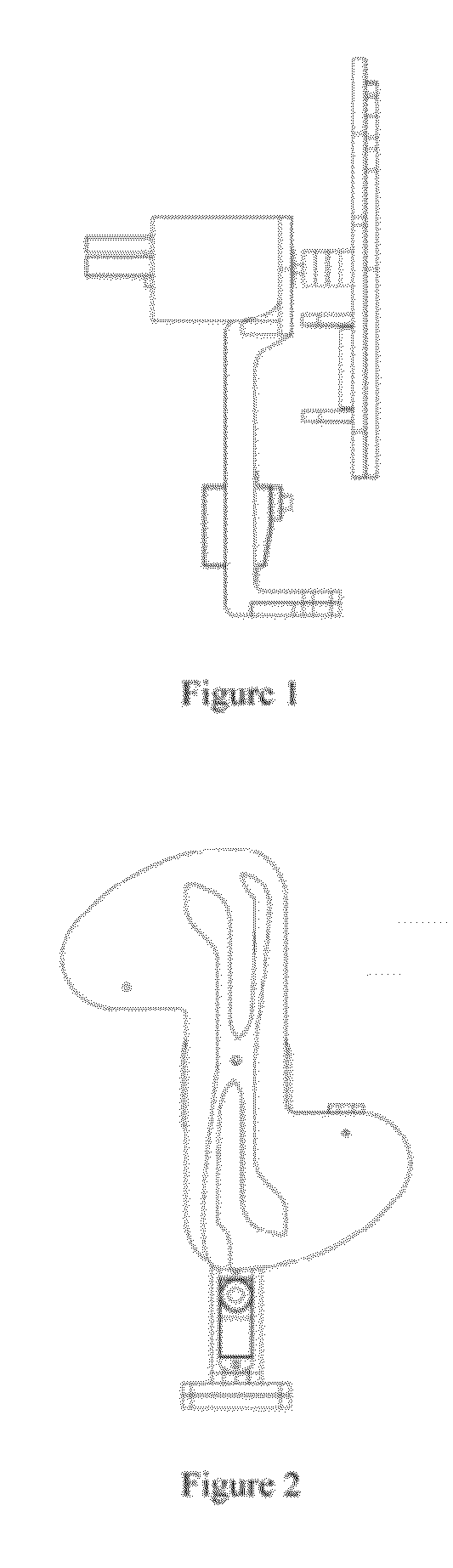
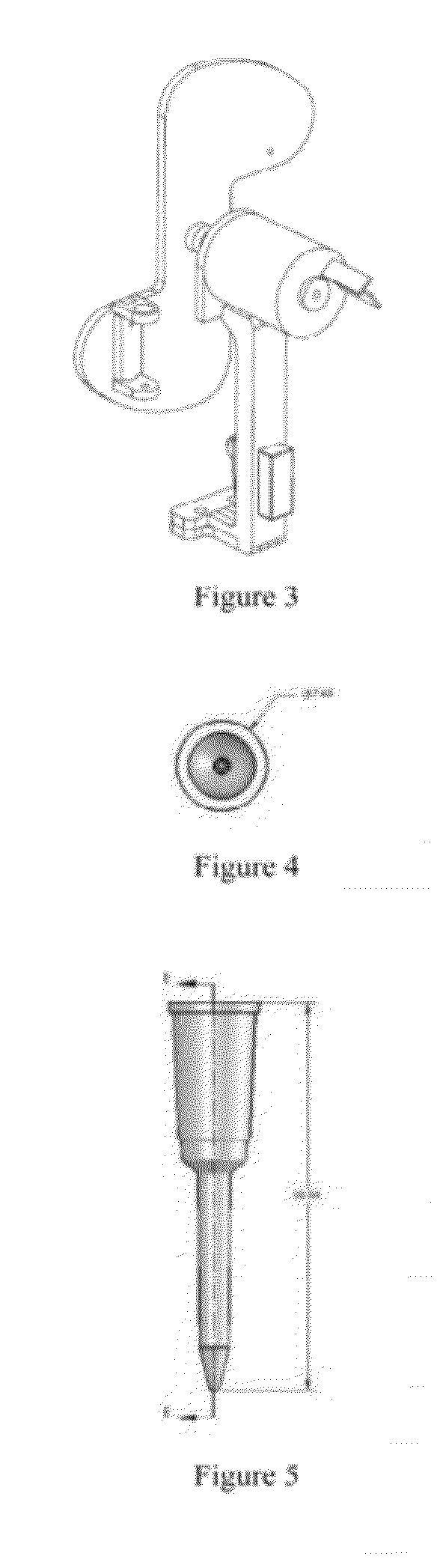
Systems and methods for sample use maximization
a sample and maximization technology, applied in the field of system and method for maximization of sample, can solve the problems of high cost of manufacturing the components of the device, inability to manufacture a poc device, and inability to perform parallel assays, and achieve the effect of high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example protocols
[0337]Many variations of protocol may be used for centrifugation and processing. For example, a typical protocol for use of the centrifuge to process and concentrate white cells for cytometry may include one or more of the following steps. The steps below may be provided in varying orders or other steps may be substituted for any of the steps below:[0338]1. Receive 10 uL blood anti-coagulated with EDTA (pipette injects the blood into the bottom of the centrifuge bucket)[0339]2. Sediment the red and white cells by centrifugation ([0340]3. Measure hematocrit by imaging[0341]4. Remove plasma slowly by aspiration into the pipette (4 uL corresponding to the worst case scenario [60% hematocrit]) without disturbing the cell pellet.[0342]5. Re-suspend the pellet after adding 20 uL of an appropriate cocktail of up to five fluorescently labeled antibodies1 dissolved in buffered saline+BSA (1 mg / mL) (total reaction volume about 26 uL2). 1Concentration will be adjusted appropriately to deal wit...
example 1
Nucleic Acid Amplification by Loop-Mediated Isothermal Amplification (LAMP)
[0791]To evaluate the ability of the three-color image analysis method for both fluorescence and absorption to read LAMP assays the following experiments were performed.
[0792]Lamp Reaction Conditions
[0793]The LAMP reaction was carried out in a total volume of 25 μL in 500 uL PCR tubes (VWR, West Chester, Pa.). The reaction mixture included 0.8 μM of primer 1 and primer 2, 0.2 μM of primer 3 and primer 4, 400 μM each dNTP (Invitrogen, Carlsbad, Calif.), 1M betaine (Sigma, St. Louis, Mo.), 1× Thermopol Buffer (New England Biolabs, Ipswitch, Mass.), 2 mM MgSO4 (Rockland Immunochemicals, Gilbertsville, Pa.), 8U Bst DNA polymerase large fragment (New England Biolabs, Ipswitch, Mass.), and a given amount of template DNA (varied between ˜10 and ˜10̂9 copies). In the case of negative control approximately 10̂9 copies of irrelevant DNA was added.
[0794]Reaction Conditions
[0795]The reaction was incubated at 65° C. for 1...
example 2
System Maximizing Sample Utilization
[0806]A system for maximizing sample utilization can have the following characteristics:
[0807]1. Efficient separation of blood into plasma and efficient recovery of the plasma[0808]a. Separation is achieved by centrifugation in a capillary tube
[0809]2. Dilution of the plasma to a few pre-established levels appropriate to both high and low sensitivity assays
[0810]3. Minimizing the volume of each assay reaction mixture required for each assay[0811]a. Using an open-ended low volume cuvette suitable for assay incubations while precluding evaporation[0812]i. Cuvette is long relative to width[0813]b. Within said low volume cuvettes enabling increase in assay signal sensitivity by modifying the optical pathlength[0814]i. Cuvette is conical or has features where the width is wide and narrow[0815]c. When needed, achieving said increase in assay signal sensitivity by moving the reaction product (which does not fill the cuvette) to selected locations having ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com