Compositions and methods for detecting or eliminating senescent cells to diagnose or treat disease
a technology of senescence cells and senescence, applied in the field of senescence cells to diagnose or treat diseases, can solve the problems of cellular senescence, increased cancer incidence and severity, and questionable whether replicative senescence actually contributes to aging or age-related symptoms in vivo, so as to reduce the size of tumors or the number, slow or prevent the growth of cancer cells, and stabilize the effect of cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Discovery of Agents that Bind Senescent Cells
Phage Selection Technique
[0133]Normal skin cell line CCD-1070Sk was obtained from American Type Culture Collection (Bethesda, Md.). The cells were grown in Eagle's Minimal Essential medium with Earle's BSS, 2 mM L-glutamine, 1.0 mM Sodium pyruvate, 0.1 mM nonessential amino acids and 1.5 g / L sodium bicarbonate supplemented with 10% fetal bovine serum.
[0134]Cells were sub-cultured every 4-5 days till they became senescent. Cell senescence was confirmed by senescence-associated beta-galactosidase staining.
[0135]An M13 phage peptide library, Ph.D.-12, was obtained from New England BioLabs (Beverly, Mass.), which displays random 12-mer peptides.
[0136]For the selection step for round 1, an aliquot (104) of the Ph.D.-12 complete phage library was incubated with 5×105 cells of senescent fibroblasts in 1 mL PBS / 0.5% BSA for ˜3.5 hours at room temperature with slow shaking on Lab-Quake. At the end of the incubation, the cells were pelleted in a mi...
example 2
Human Study to Test the Prognostic Use of a Senescent Cell Binding Agent Labeled with a Radioisotope; Using a Senescent Cell Detecting Radiotracer to Predict Cancer Risk
[0140]This study is designed to show that noninvasive, in vivo imaging of senescent cell content can be used to predict cancer risk in smokers. Six-hundred subjects will be enrolled. Eighty percent of these subjects will be smokers; twenty percent will be nonsmokers. Subjects less than 18 years of age, subjects with a history of cancer, and pregnant subjects will be excluded. The anticipated mean age of study subjects is 55 years. Each study subject will undergo scintigraphic imaging using a radio-labeled peptide of the invention (SEQ ID NO:1) as the radiopharmaceutical. The radiopharmaceutical will be prepared by reacting peptide-gly-gly-gly-ser-DTPA with 111-In chloride. Each study subject will receive an intravenously administered dose of 5 mCi of 111-In labeled peptide. Anterior and posterior whole body planar im...
example 3
Using Senescent Cell Binding Agents Coupled to Cytotoxic Agents to Eliminate Senescent Cells: Effect on Subsequent Development of Cancer
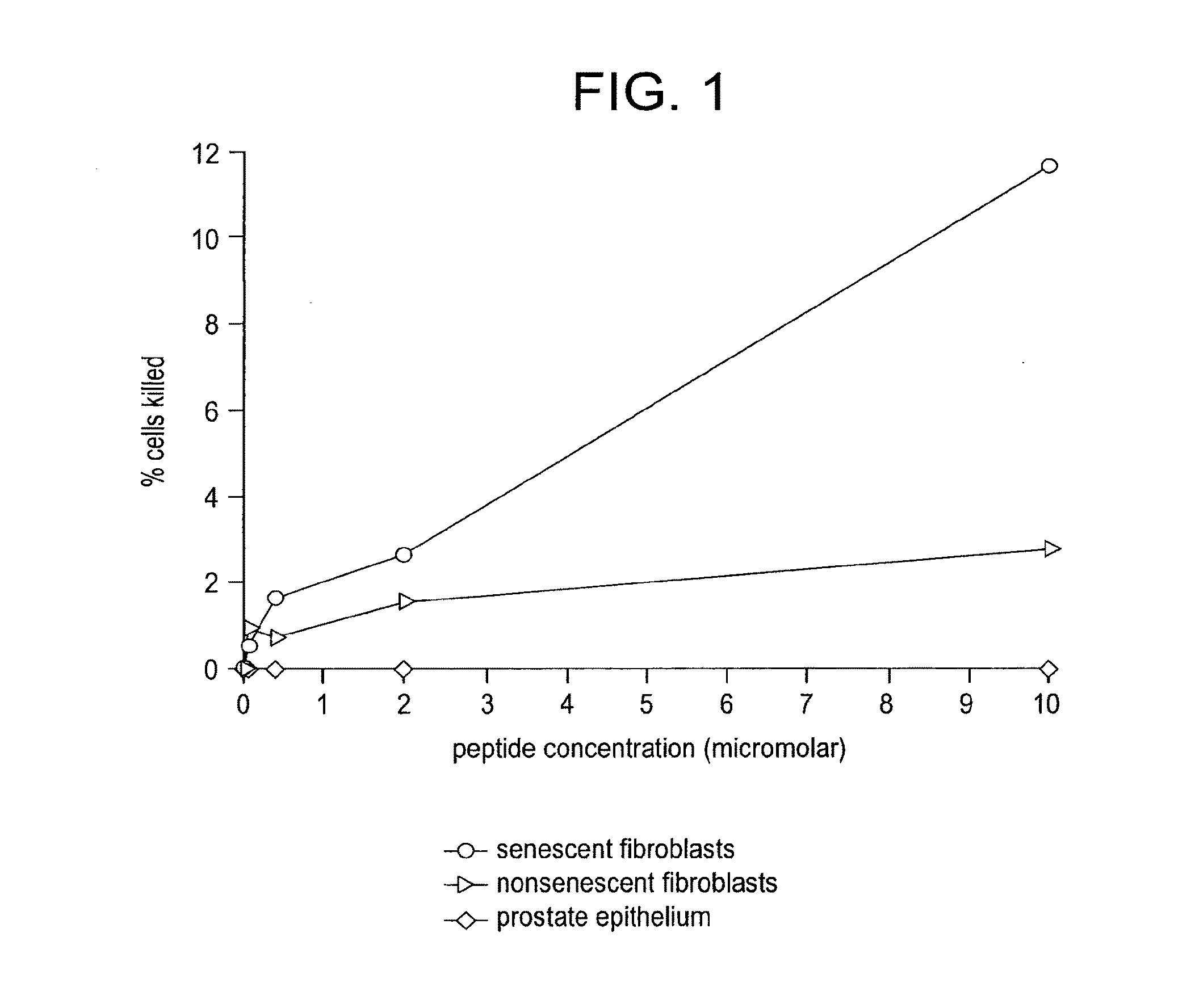
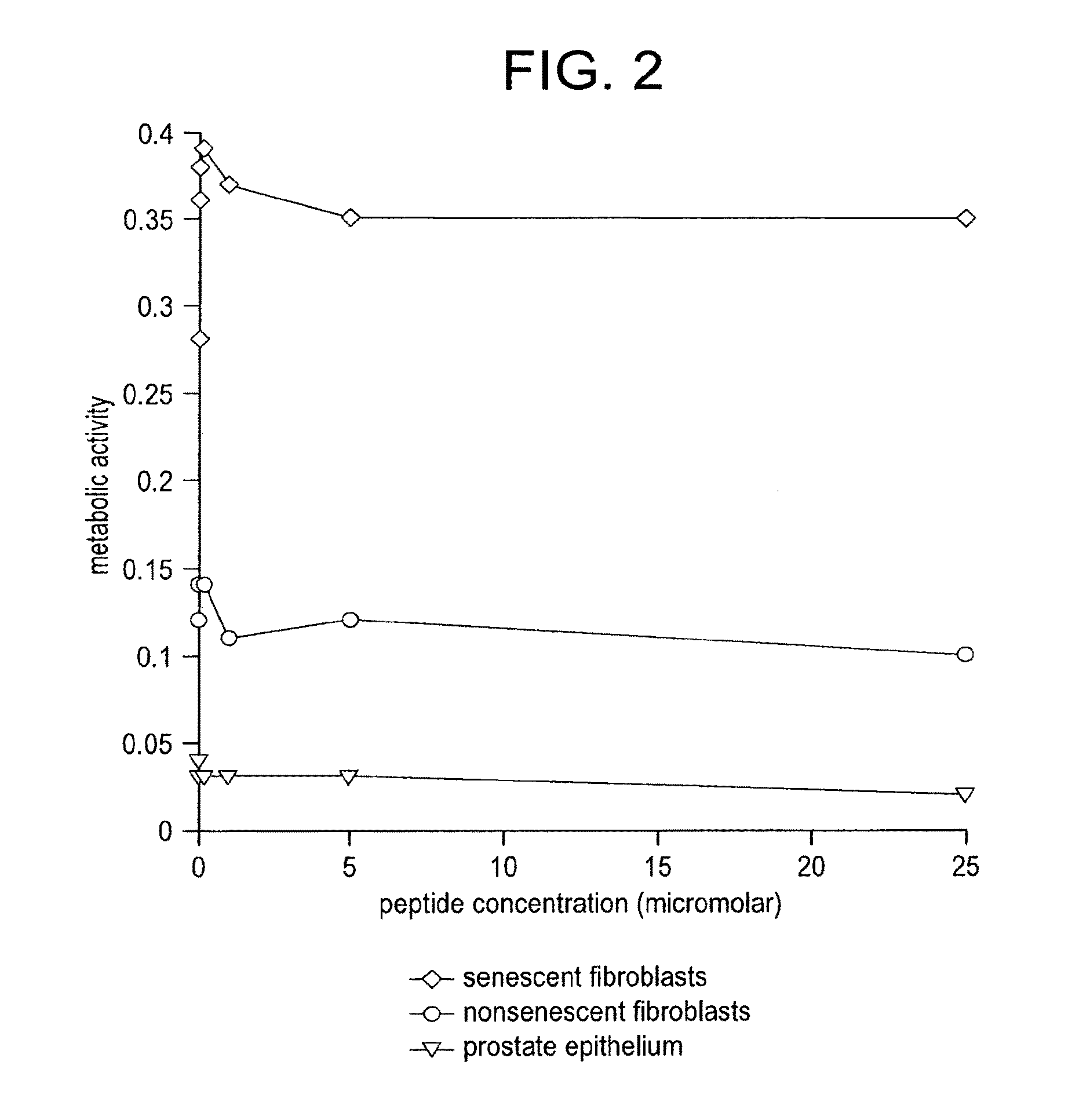
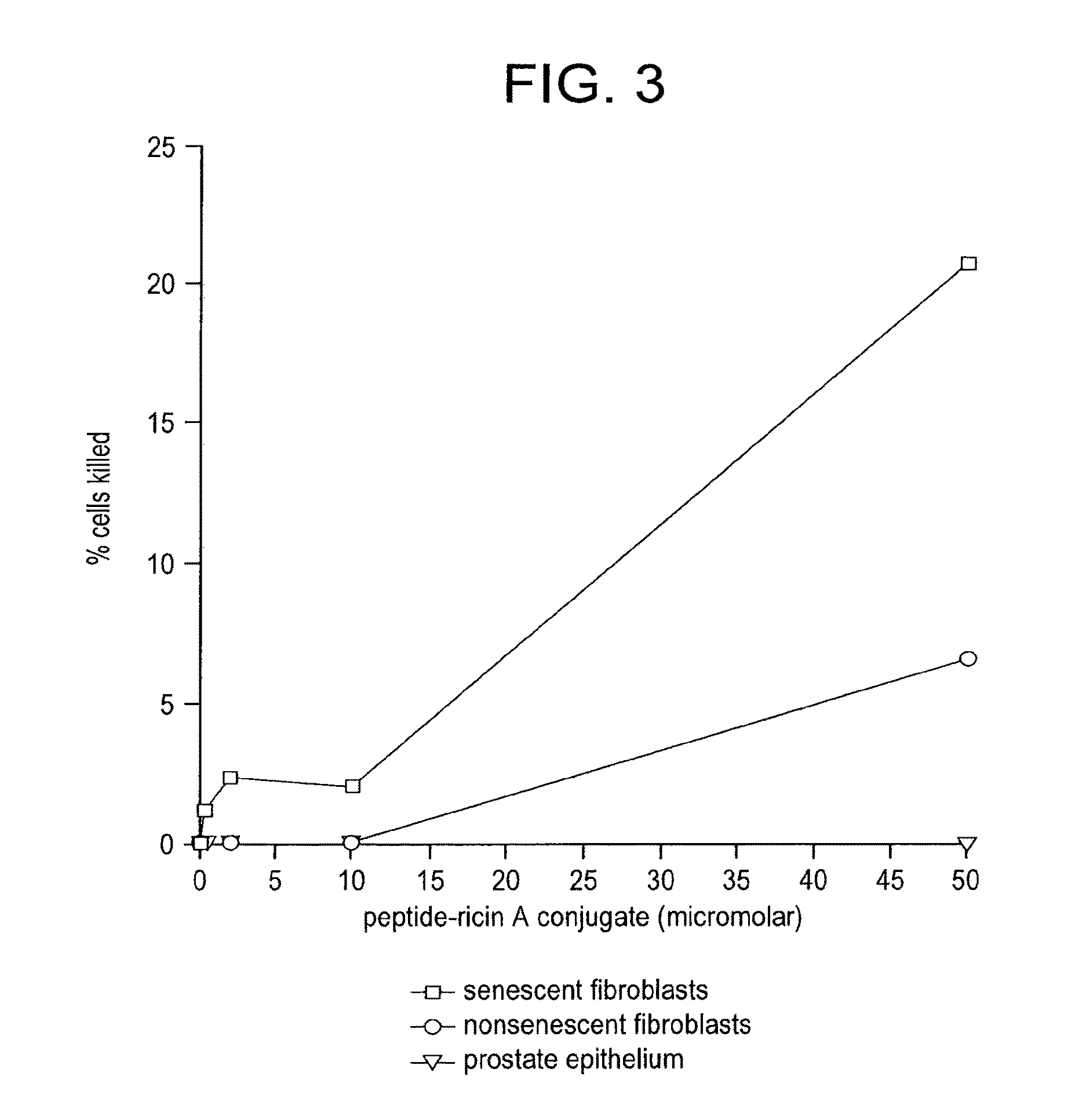
[0141]The purpose of this study is to show that elimination of some senescent cells from an organism using the agents of the invention (e.g., peptides, polypeptides, proteins, small molecules, antibodies, or antibody fragments that target senescent cells) will reduce the risk of subsequently developing cancer. For example, a senescent cell binding peptide. (SEQ ID NO:1) will be conjugated to a cytotoxic peptide having the sequence KFAKFAKKFAKFAKKFAKFAK (SEQ ID NO:4; Leuschner and Hansel, Biology of Reproduction 73:860-865) via an amino acid linker sequence (e.g., a linker sequence selected from GGGC (SEQ ID NO:9), GGGS (SEQ ID NO:10), and GG) at the C terminus of the senescent cell binding peptide. Study subjects will consist of 60 BALB / c mice, mean age 6 mo which includes 30 experimental animals and 30 controls. Experimental animals will receive an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dissociation constant | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com