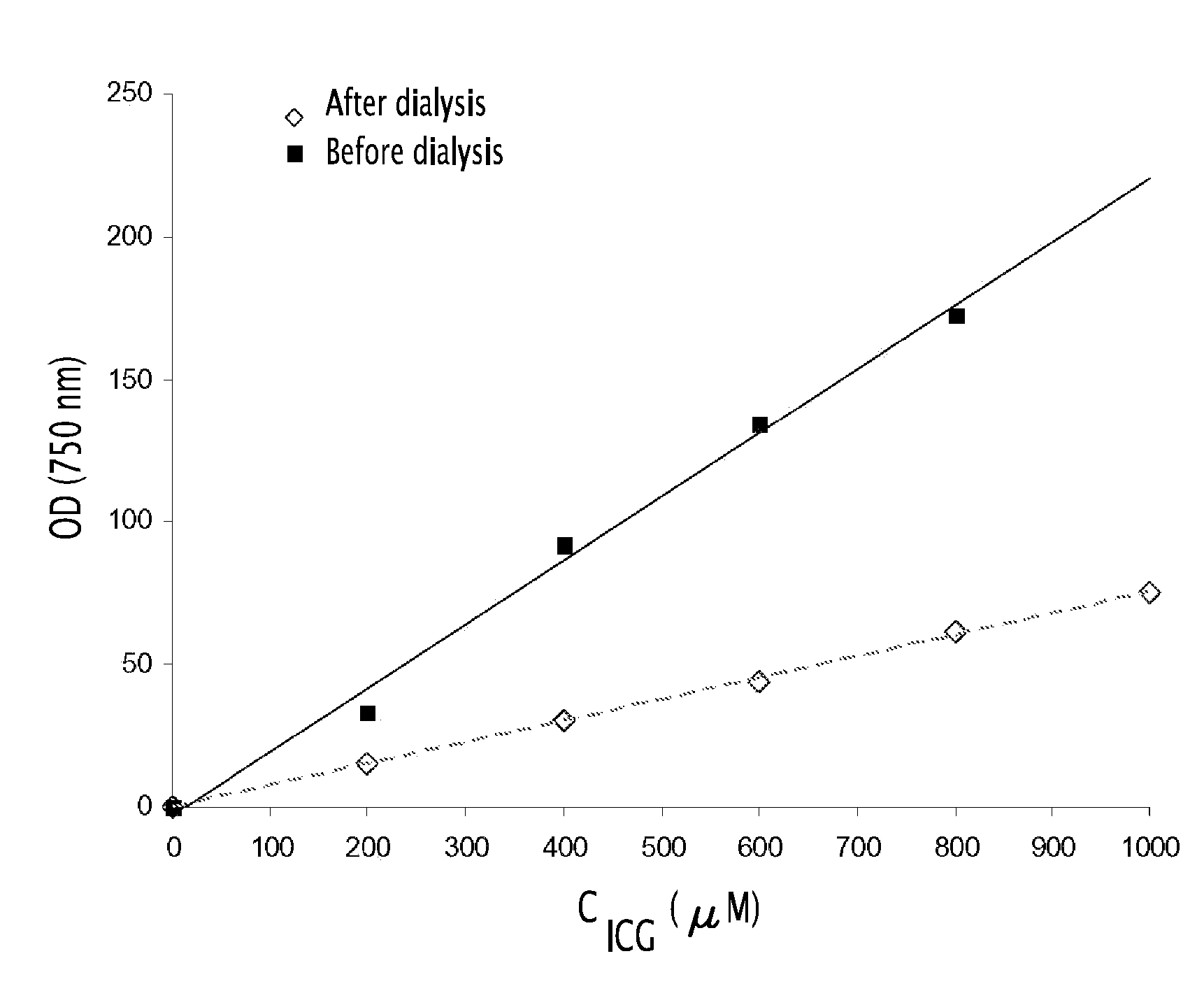
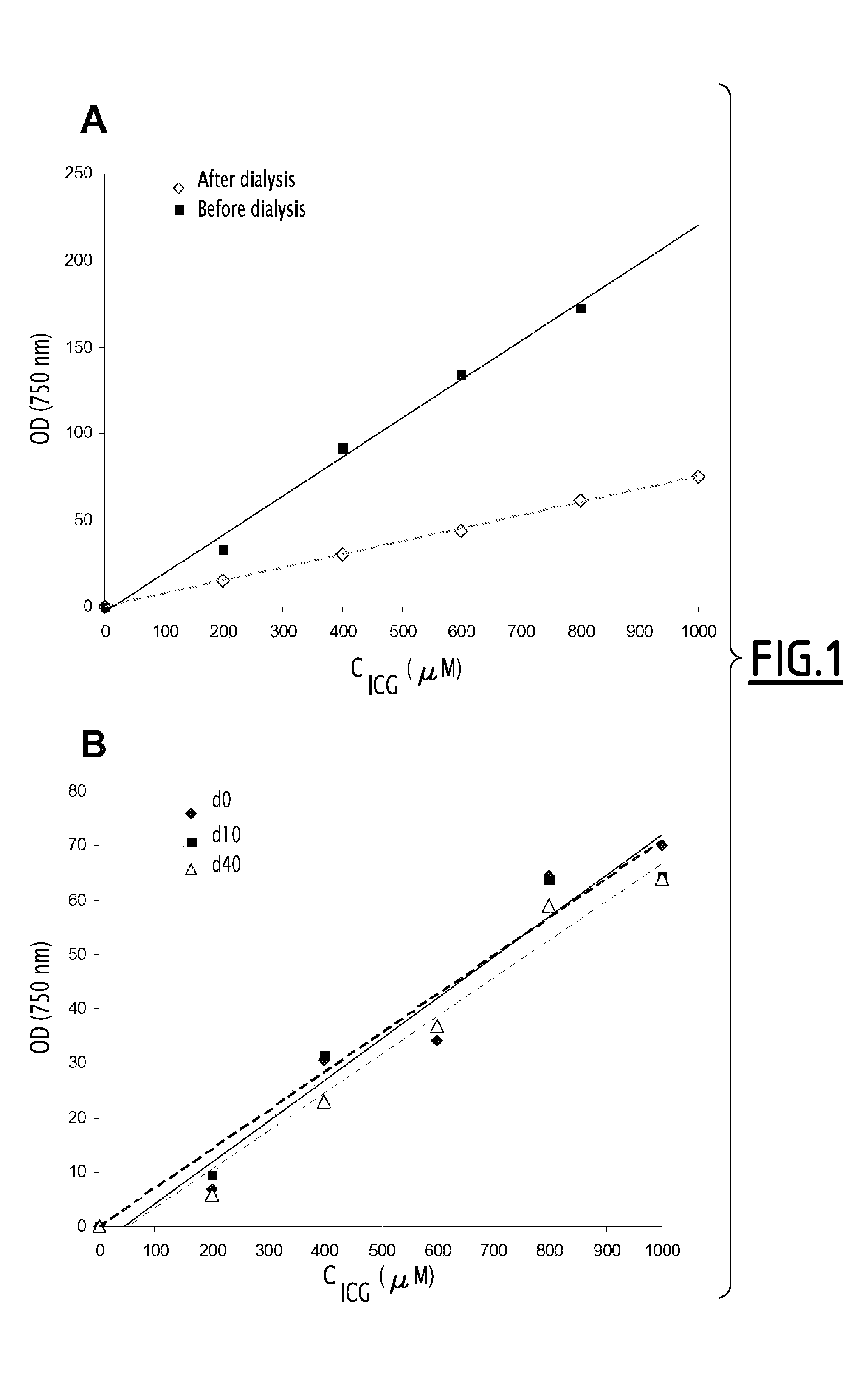
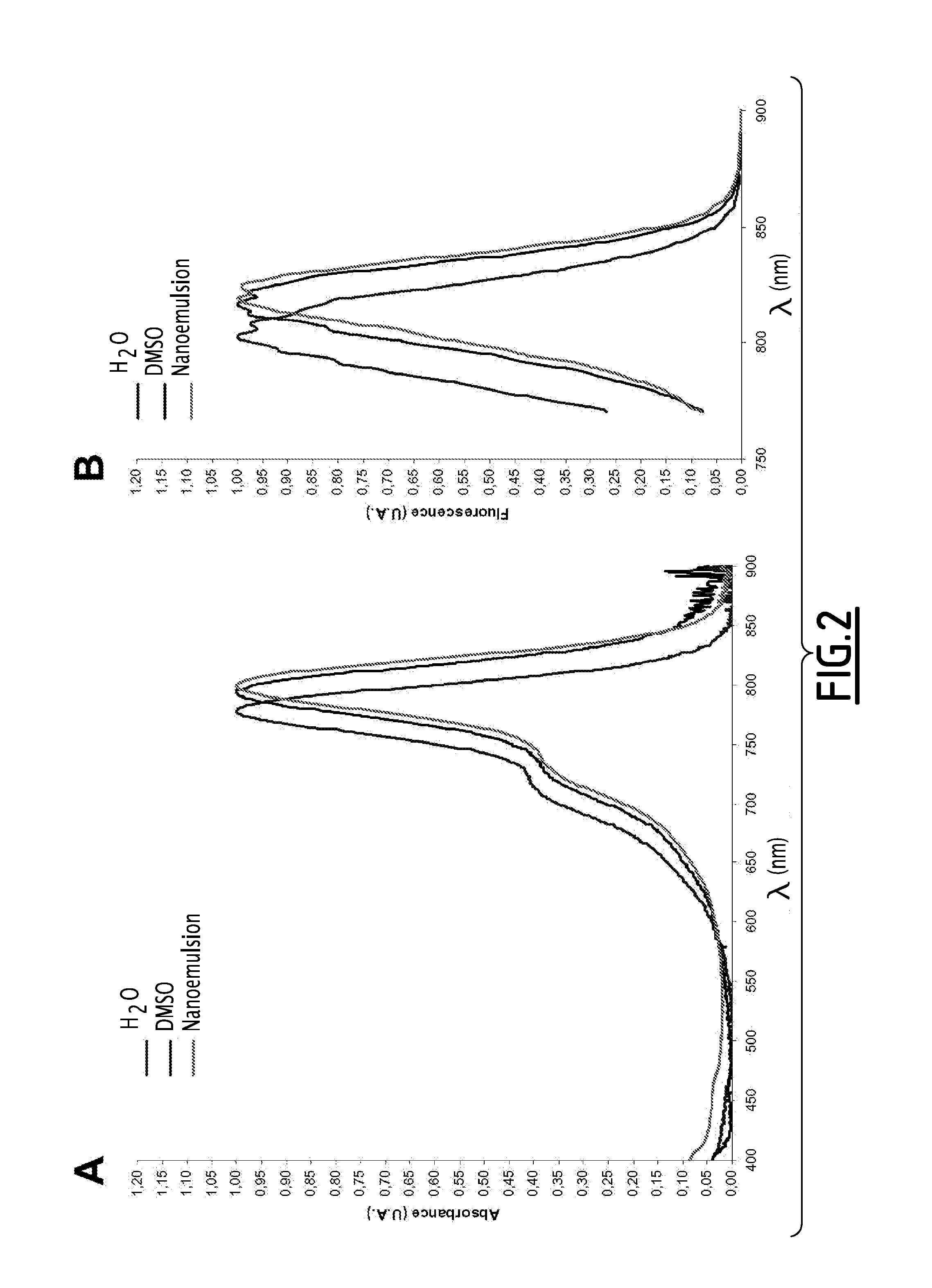
Fluorescent emulsion of indocyanine green
a technology of indocyanine green and fluorescence, which is applied in the field of fluorescence emulsion of indocyanine green, can solve the problems of poor soluble in water, difficult use of indocyanine green fluorescent labels, and some properties of indocyanine green, so as to improve imaging performance, improve optical characteristics, and improve performan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Formulation of ICG in Emulsion Form
[0134]In a suitable container there was prepared a premixture constituted by 0.05 g of soybean oil (Sigma-Aldrich), 0.150 g of semi-synthetic glycerides sold under the trade name Suppocire® NC (Gattefossé) and 0.310 mg and 9.30 mg of ICG (Cardiogreen, Sigma-Aldrich or Infracyanine Serb laboratoires) in solution in dimethylsulfoxide (DMSO) as well as 0.100 g of soybean lecithin (enriched with 45% phosphatidylcholine) sold by Lipoïd under the trade name Lipoïd® S45.
[0135]After evaporation of the DMSO in vacuo, the residue is heated to 50-60° C. and the liquid mixture is maintained at that temperature for the emulsification (at ambient temperature the mixture becomes waxy).
[0136]The continuous phase was prepared by mixing 0.05 g of glycerol, 0.331 g of polyoxyethylene stearate having 50 ethylene oxide units, sold under the trade name Myrj® 53 by ICI Americas Inc., and 154 mM sodium chloride solution to make the mixture up to 1.7 g. The solution was th...
example 2
Imaging of Vascularisation
[0149]Male rats of the strain Sprague Dawley (Harlan France) are anaesthetised by isoflurane inhalation (4% for induction and 2% for maintenance) and then placed beneath an imaging device. The device is constituted by: 1) a light source emitting in the excitation band of ICG and the excitation power of which is of the order of several mW / cm2 and 2) a CCD camera coupled to a lens adapted to the observed sample. The assembly is equipped with filters which allow the excitation light and flare to be avoided and only the fluorescence light to be collected. Dissection of the region of the neck is then carried out in order to isolate the carotid and the jugular vein. An ICG formulation prepared as described in Example 1 with a load rate of 350 μM after dialysis is then injected intravenously. Fluorescence imaging of the vascular system in that region is then carried out with visualisation firstly of the carotid and then of the jugular vein as shown in FIG. 7. This...
example 3
Imaging of Sentinel Nodes
[0150]Female mice of the strain Nude (Janvier) are anaesthetised by isoflurane inhalation (4% for induction and 2% for maintenance) and then placed beneath the fluorescence imaging device described above.
[0151]10 μA of an ICG formulation (0.5 nmole injected) prepared as described in Example 1 or of ICG dissolved in glucose water (1 nmole injected) are injected intradermally into the right rear paw.
[0152]Temporal monitoring of the mice which had received the injections was then carried out by fluorescence imaging. It is noted that the ICG tracer formulated as nanoemulsions accumulates rapidly (from the first 5 minutes) and preferentially in the lymph nodes close to the injection site. In addition, the use of the ICG tracer formulated as nanoemulsions permits access to a more sensitive detection of the lymph nodes as compared with the results obtained using ICG formulated in 5% glucose water, as shown in FIGS. 8A and 8B.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com