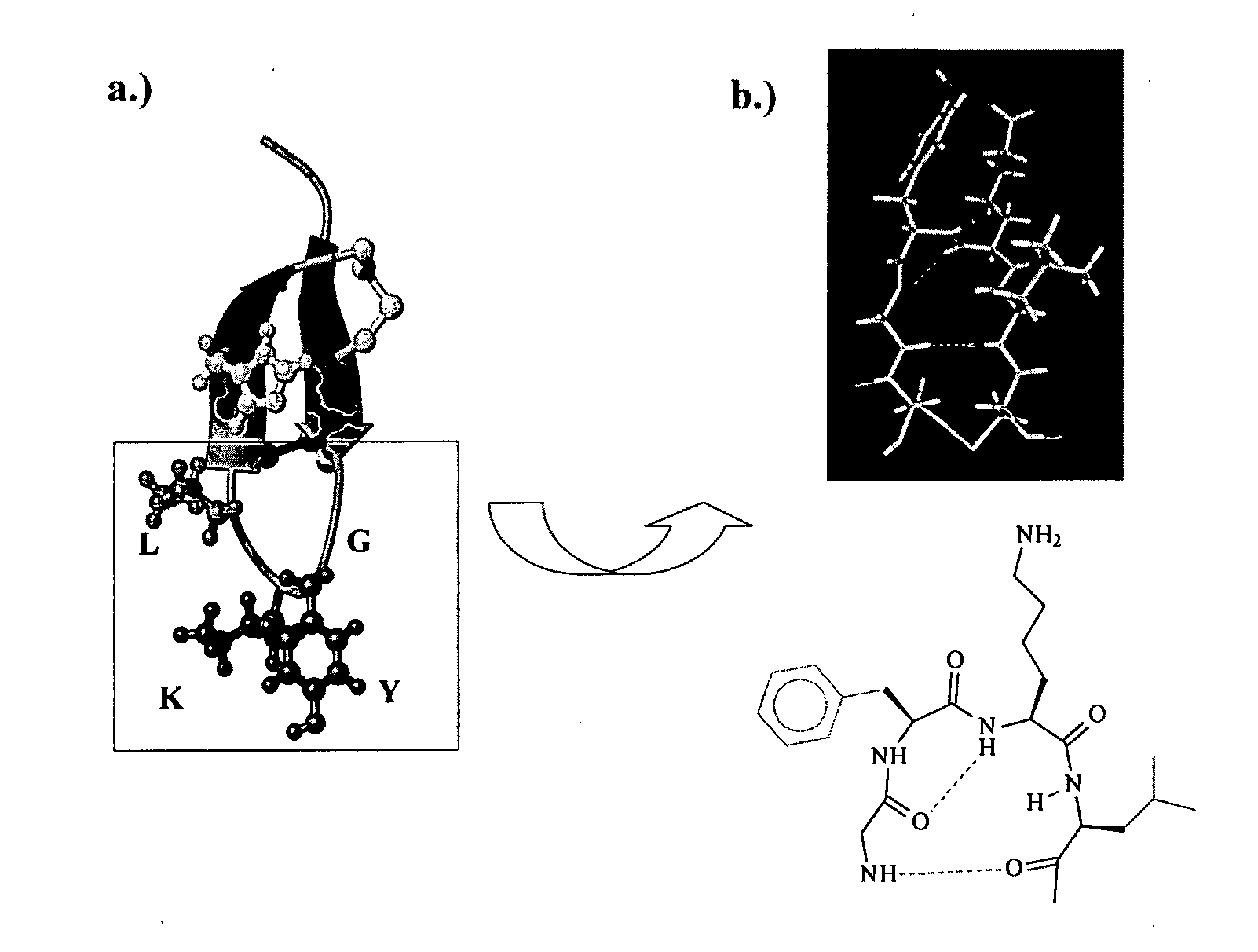
Libraries of peptide conjugates and methods for making them
a technology of conjugates and libraries, applied in the field of peptide conjugates, can solve the problem that the supply of suitable compounds for assay becomes a rate-limiting step
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
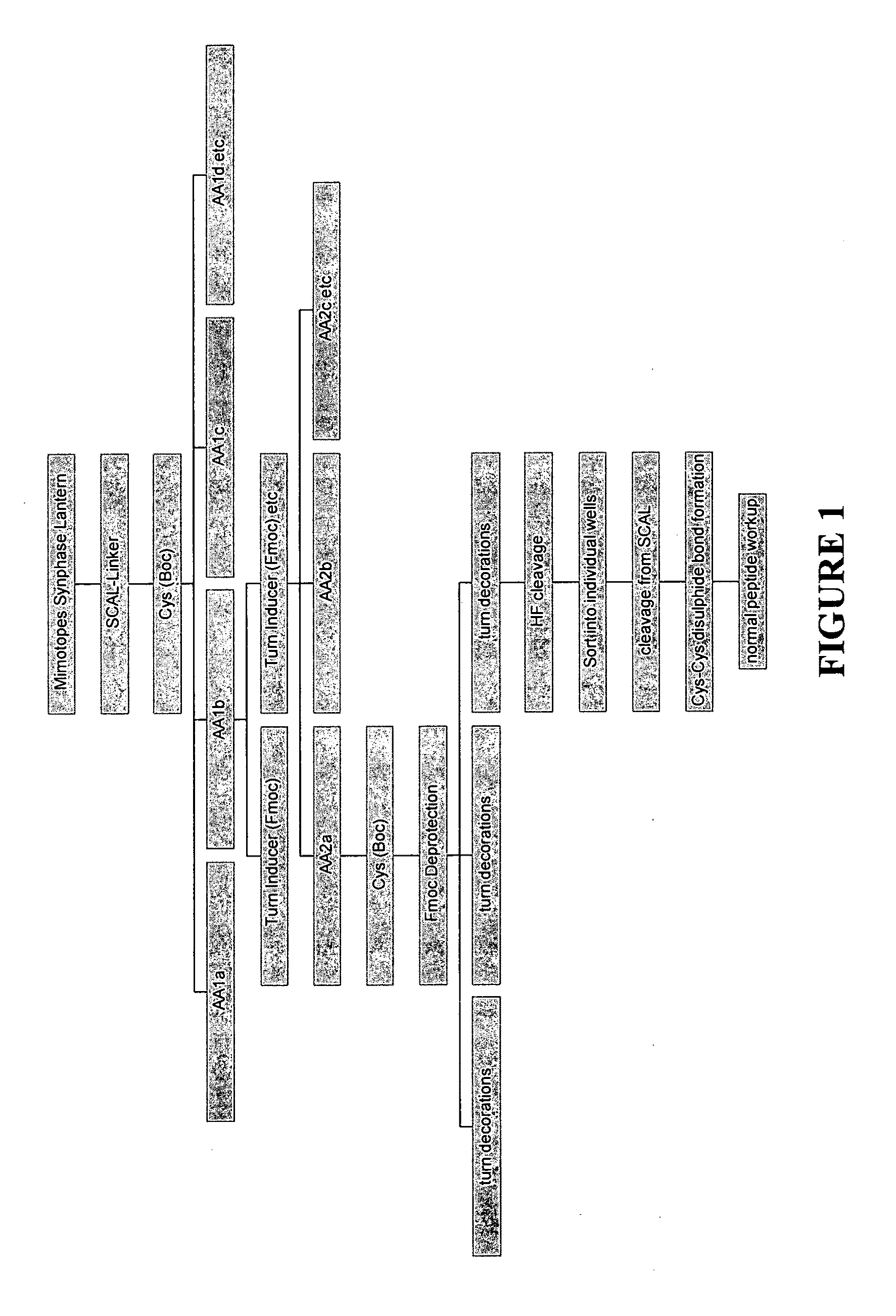
example 1
Preparation of a Library of Cyclized Peptide Conjugates
Reagents:
[0267]Protected BOC-amino acid derivatives were purchased from Auspep P / L (Melbourne, Australia). The following side chain protected BOC-amino acids were used: Cys(Mbzl), Val, Ile, Leu, Met, Phe, Tyr(2BrZ), Ser(Bzl), Thr(Bzl), Asn(Xan), Gln(Xan), Asp(OcHx), Glu(OcHx), Lys(2C1Z), Arg(Tos), His(Tos). Turn inducer (2S,4S)-Fmoc-4-amino-1-BOC-pyrrolidine-2-carboxylic acid, (2S,4R)-Fmoc-4-amino-1-BOC-pyrrolidine-2-carboxylic acid and (2S,4S)-Boc-4-amino-1-Fmoc-pyrrolidine-2-carboxylic acid as well as Fmoc-4-amino-butyric acid was purchased from NeoMPS (Strasbourg, France). Dimethylformamide (DMF), dichloromethane (DCM), diisopropylethylamine (DIEA), Trifluoroacetic acid (TFA) were all peptide synthesis grade supplied by Auspep P / L (Melbourne, Australia). Benzoic acid, 2-naphthoic acid, 4-hydroxy-benzoic acid, cyclohexyl acetic acid, nicotinic acid, succinic acid anhydride, isovaleric acid, p-cresol, Ammonium Iodide, dimethyls...
example 2
Preparation of a Library Using Dithioether Cyclization to Form Methylendithioether Peptide Conjugates
[0283]Similar methods used in Example 1 are used with the following variations. Lanterns (30) obtained from. HF cleavage are covered with a solution of 6 g tetrabutyl ammonium fluoride hydrate in DCM (20 mL) for a period of 18 h. The lanterns than are washed multiple times with DCM and then dried in vacuum. The obtained dithioether peptides are then treated as described in example 1 to obtain SCAL linker cleavage with the exception that a final DMSO oxidation is not required. The workup is identical to that described in Example 1. The method used is depicted in FIG. 3B.
example 3
Library Validation
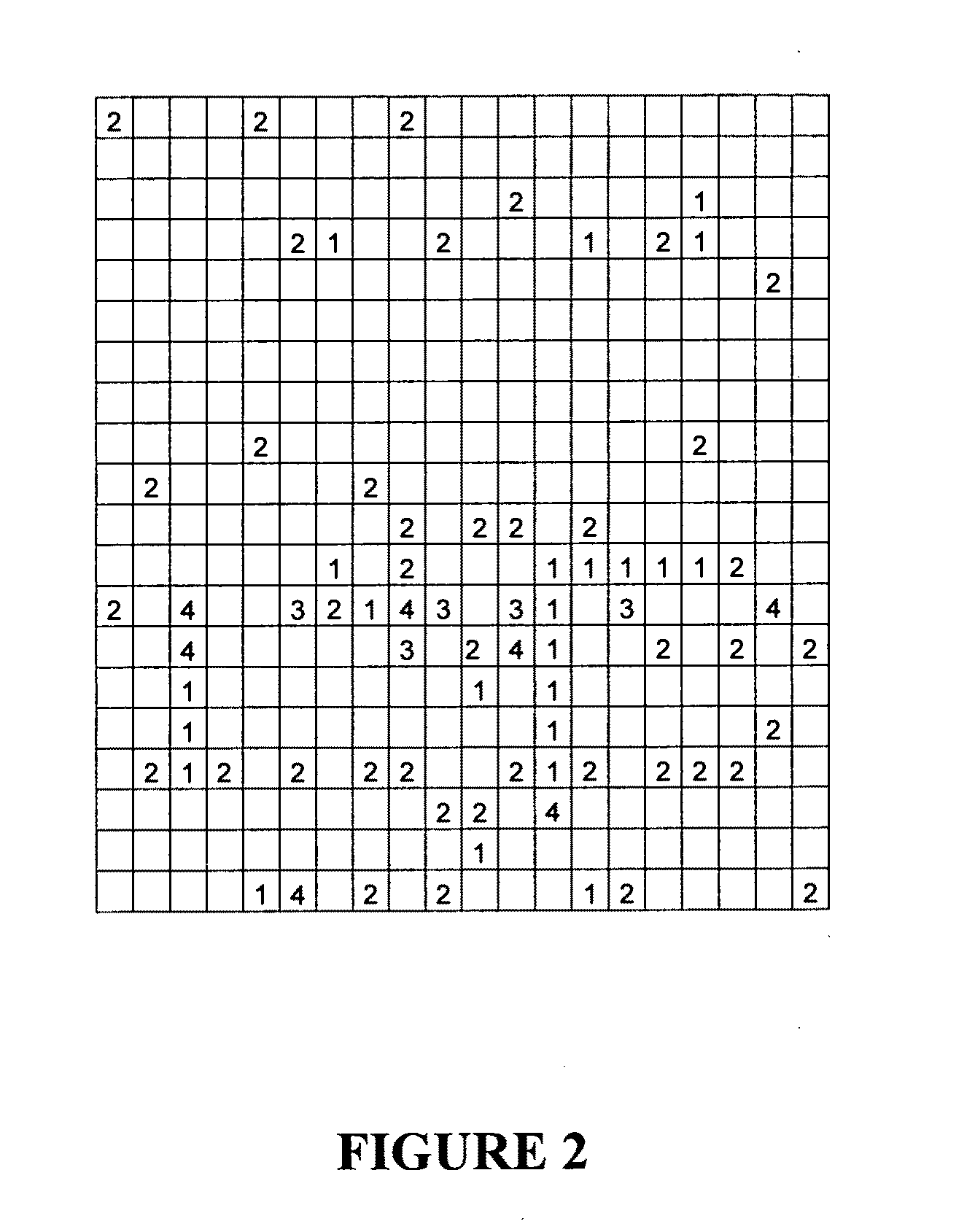
[0284]Using the methods described in Examples 1 and 2, a library of 5400 peptide conjugates was constructed according to formula I, with a range of variants for A, R1, R2 and R3 thereby representing significant structural and chemical diversity. The peptide conjugates were plated in a format suitable for high or medium throughput screening.
[0285]To provide proof-of-concept for the methods and demonstrate the utility of the resulting peptide conjugates, a subset of the library (400 compounds) was tested against examples of three different classes of drug target, namely ion channels, GPCRs and transporters.
[0286]Detailed descriptions of these experiments and results are provided in Examples 4 to 7. In brief, the validation program yielded numerous hits at all three targets (FIG. 2) supporting the broad applicability of the library to a variety of target types. These screens also revealed the significant target specificity or selectivity that can be achieved with indi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com