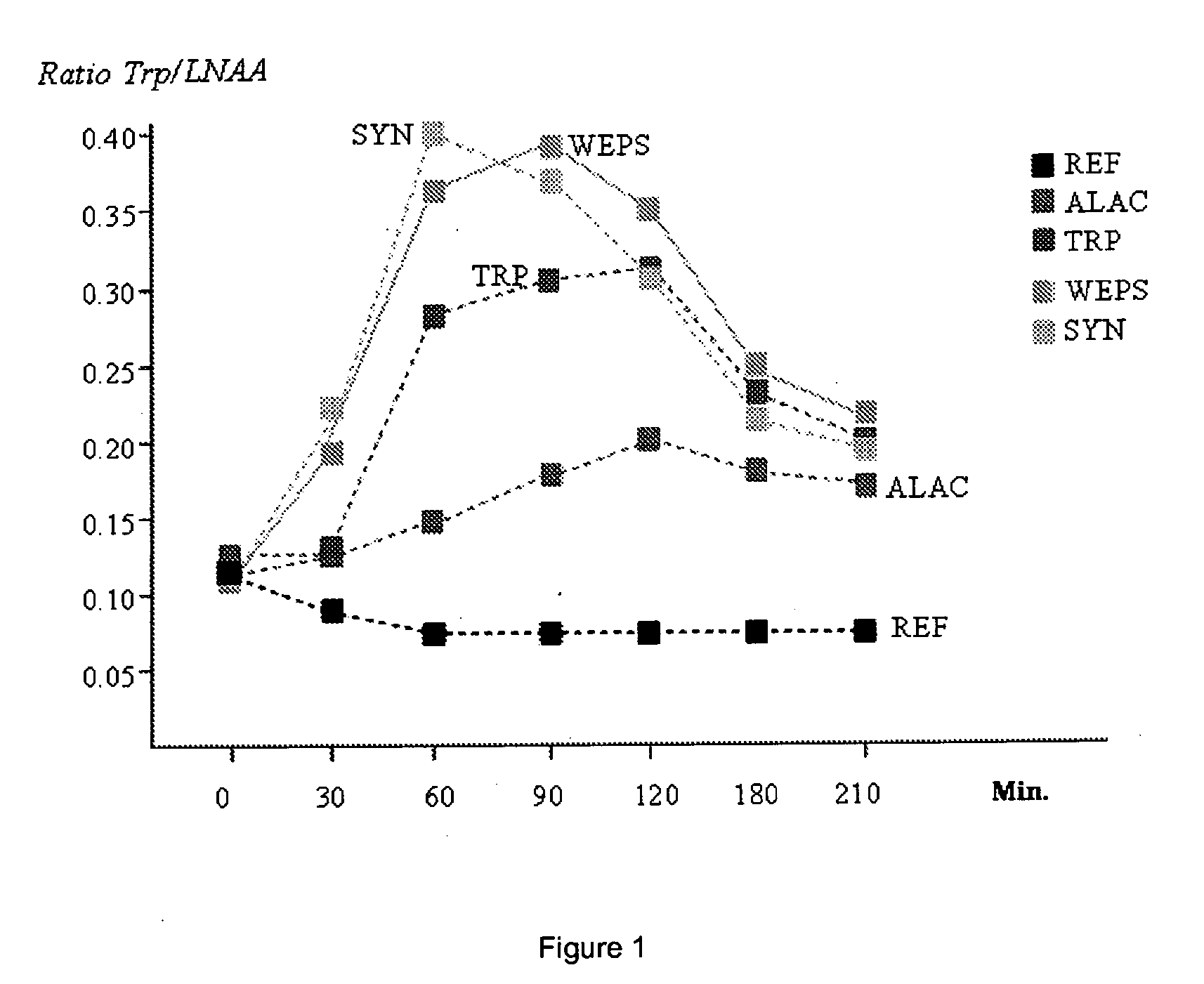
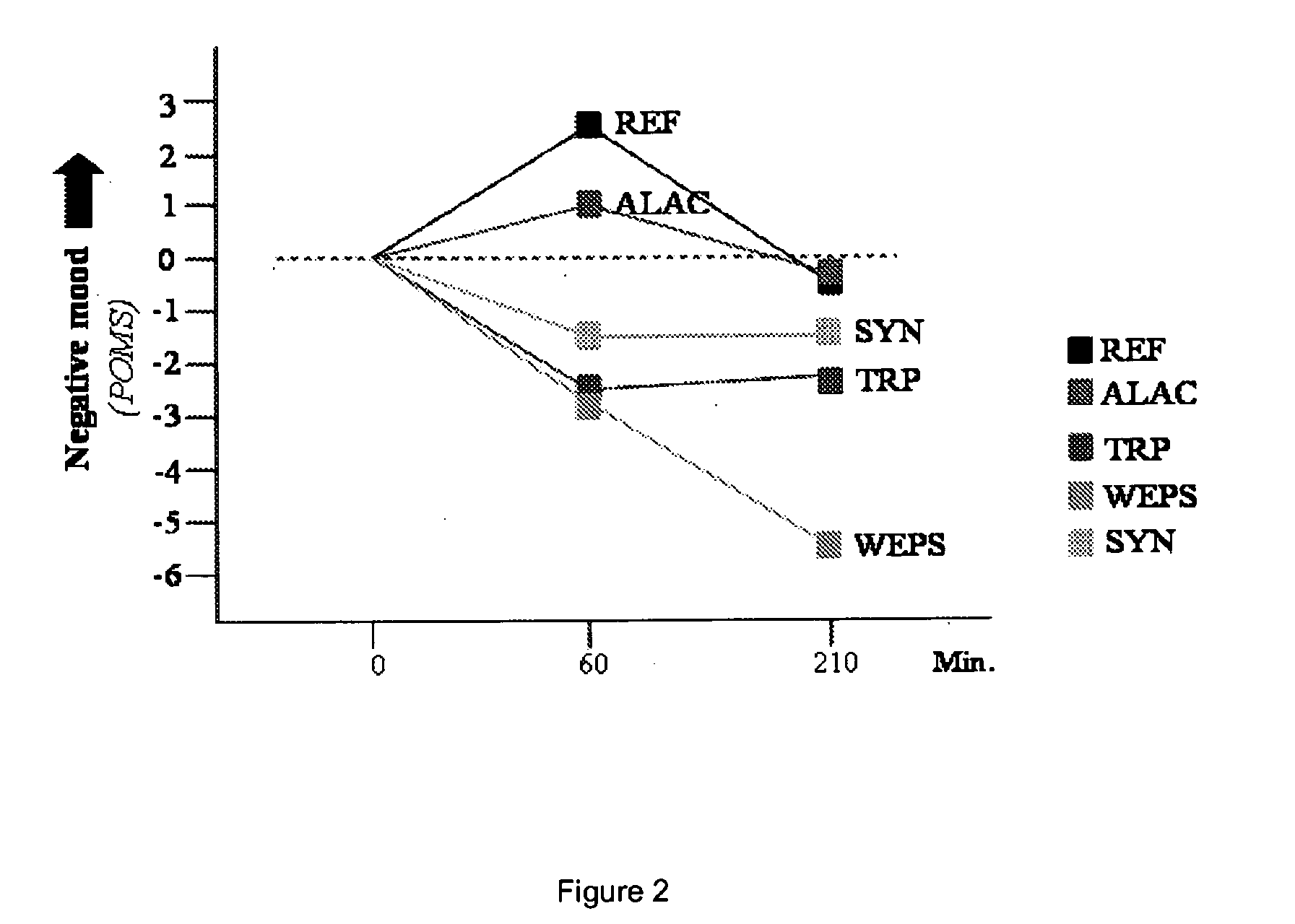
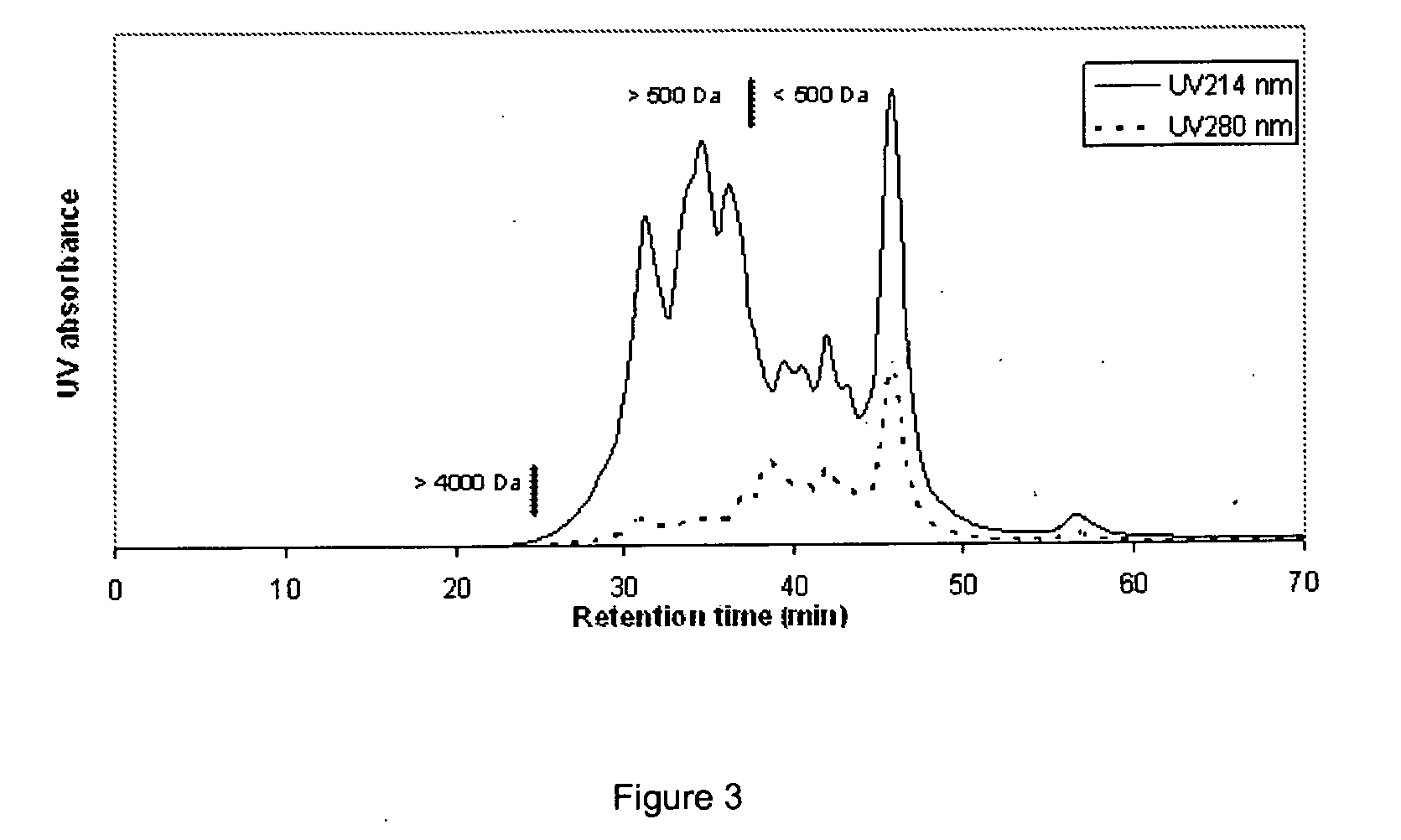
Peptides containing tryptophan
a technology of tryptophan and peptides, which is applied in the direction of peptide/protein ingredients, drug compositions, biocides, etc., can solve the problems of difficult complete separation of alpha-lactalbumin, inability to fully separate alpha-lactalbumin, and inability to achieve the maximum trp/lnaa ratio and cost, etc., to improve alertness, mood, cognition or sleep patterns, and relieve stress or depression.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Hen Egg Lysozyme is not Cleaved by Either Pepsin or Trypsin / Chymotrypsin
[0078]To test its digestibility in the human gastrointestinal tract, hen egg lysozyme was incubated in vitro with pepsin as well as with a mixture of trypsin and chymotrypsin. Both incubations were carried out under pH conditions that are prevalent in the stomach (pepsin) and duodenum (trypsin / chymotrypsin). To that end, a 5% (w / w) lysozyme solution was incubated with the enzymes (1% w / w enzyme to lysozyme protein) for 2 hours at 37 degrees C. To prevent major pH changes as the result of the ongoing protein hydrolysis, incubation was carried out in a Mc Ilvane buffer (0.2 M citric acid plus Na2HPO4). The low DH's values that are obtained after the two hours hydrolysis at 37 degrees C. (see Table 1), demonstrate that the lysozyme molecule cannot be degraded under conditions that mimic digestion conditions in the stomach and in the duodenum and jejunum because successful proteolysis can be expected to lead to a DH...
example 2
Hen Egg Lysozyme is Efficiently Cleaved by Subtilisin at Elevated pH Values
[0079]To test the susceptibility of lysozyme to enzyme hydrolysis under non-physiological pH and enzyme conditions, a lysozyme solution was incubated in vitro with a microbial subtilisin (EC 3.4.21.62) under alkaline pH conditions. To that end, a 5% (w / w) lysozyme solution was incubated at pH 7.0, 8.0 and 9.0 with 12.5 microliter of Protex 6L.per gram lysozyme protein present. The incubation was carried out for 3 hours at 60 degrees C. with a constant adjustment of the pH using 1M NaOH. The incubations yielded slightly turbid solutions without any significant precipitates. After a heating step to inactivate the subtilisin activity, the DH values of the various incubations were measured according to the protocol described in the Materials& Methods section. In contrast with the results obtained under physiological conditions (see Example 1), alkaline incubation conditions using subtilisin result in complete lys...
example 3
Hydrolysing Lysozyme Using Protex and Identity of the Peptides Formed
[0080]A solution containing 10% (w / w) pure lysozyme was adjusted to pH 8.2 using NaOH and heated to 52 degrees C. Hydrolysis was started by adding 25 microliter of
[0081]Protex / g of protein present. Under continuous stirring and maintaining the pH at 8.2, the hydrolysis was continued for 5.5 hours to yield an almost clear solution without a visible precipitate. After a heating step to inactivate the Protex activity, a sample was taken for DH analysis. The DH of the solution turned out to be almost 30%. The heat treated solution was ultrafiltered over a 10 kDa filter to yield a completely clear liquid. This clear liquid was used for LC / MS analysis, for molecular weight distribution of peptides and proteins present as well as for ion exchange chromatography.
[0082]To get an impression of the molecular weight distribution of peptides and proteins present, the clear liquid was subjected to a molecular size analysis as de...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com