Ester-based peptide prodrugs
a peptide and prodrug technology, applied in the field of peptide-based prodrugs, can solve the problems of life-threatening hypoglycemia, extremely difficult to normalize blood glucose without occurrence of hypoglycemia, and demonstrated unparalleled ability to lower glucose in virtually all cases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiments
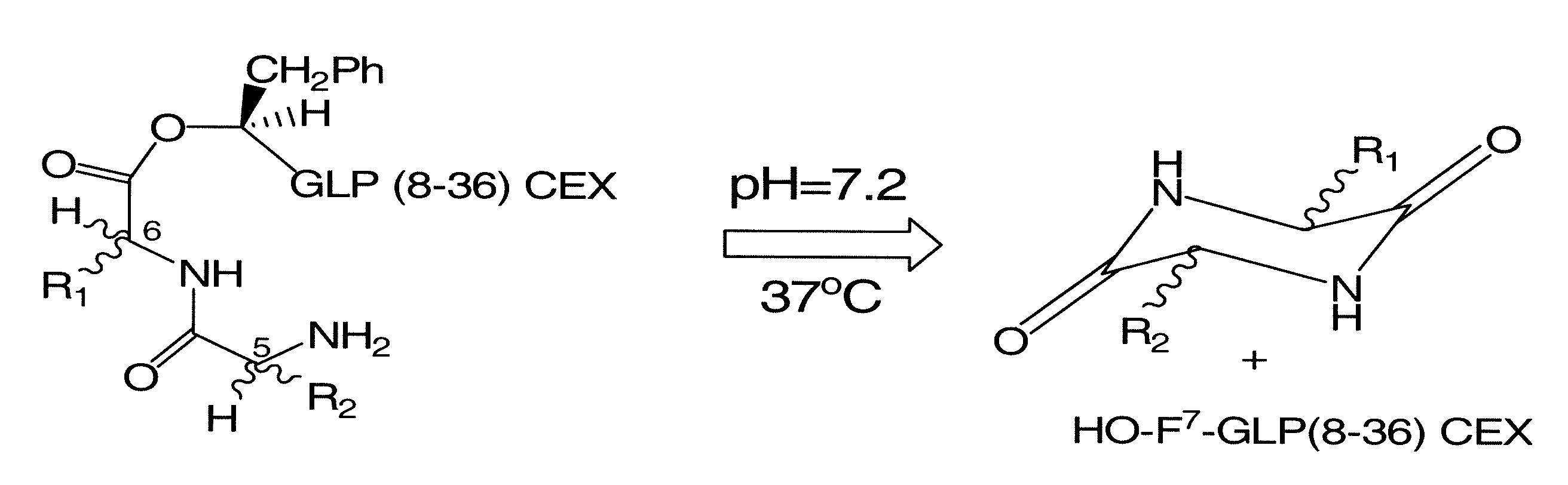
[0136]The present disclosure describes the formulation of prodrug derivatives of known bioactive polypeptides. More particularly, the prodrugs disclosed herein are formulated to enhance the half life of the parent bioactive peptide or protein, while allowing for activation of the prodrug via a non-enzymatic degradation mechanism. The ideal prodrug should be soluble in water at physiological conditions (for example, a pH of 7.2 and 37° C.), and it should be stable in the powder form for long term storage. It should also be immunologically silent and exhibit a low activity relative to the parent drug. Typically the prodrug will exhibit no more than 10% of the activity of the parent drug, in one embodiment the prodrug exhibits less than 10%, less than 5%, about 1%, or less than 1% activity relative to the parent drug. Furthermore, the prodrug, when injected in the body, should be quantitatively converted to the active drug within a defined period of time. As disclosed herein, applicant...
example 1
Synthesis of glucagon and GLP-1 analogs
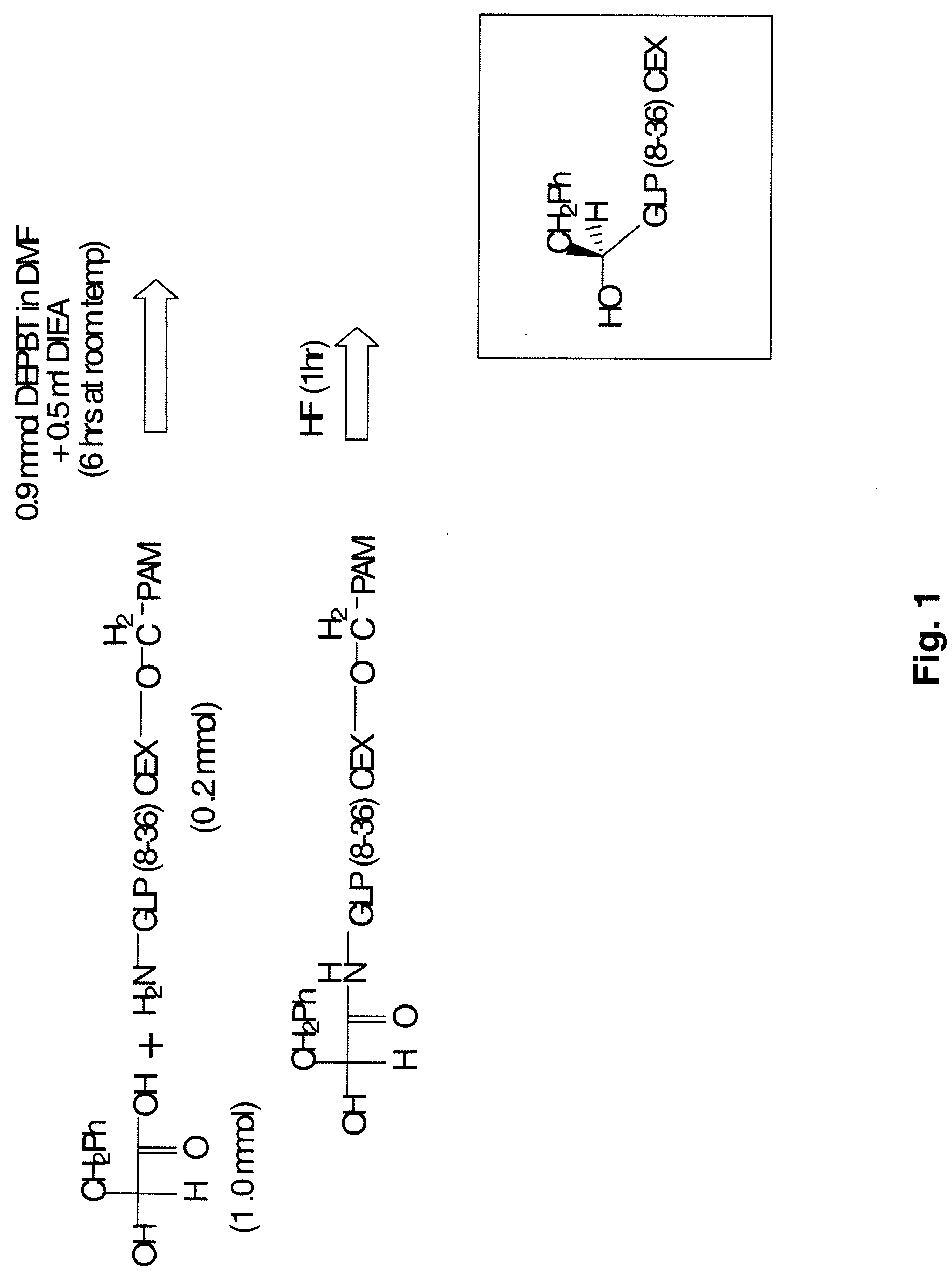
[0980]To investigate the possibility of preparing prodrugs derivative of glucagon and GLP-1, numerous peptide analogs were synthesized. The standard procedure is described briefly here and in FIGS. 3 and 4, and the details are discussed later.
[0981]Materials:
[0982]PAM resin (PAM resin is OCH2-phenylacetamidomethyl-copolystyrene-1% divinylbenzene), (100-180 mesh, 1% DVB cross-linked polystyrene; loading of 0.7-10 mmol / g), Boc-protected and Fmoc protected amino acids were purchased from Midwest Biotech. Other reagents such as the α-hydroxy-acids (phenyllactic acid and glycolic acid) were purchased from Aldrich. The solid phase peptide syntheses using Boc-protected amino acids were performed on an Applied Biosystem 430A Peptide Synthesizer. Fmoc protected amino acid synthesis was performed using the Applied Biosystems Model 433 Peptide Synthesizer. The manual synthesis of depsi-peptides was performed in sintered reaction vessels using analogous pr...
example 2
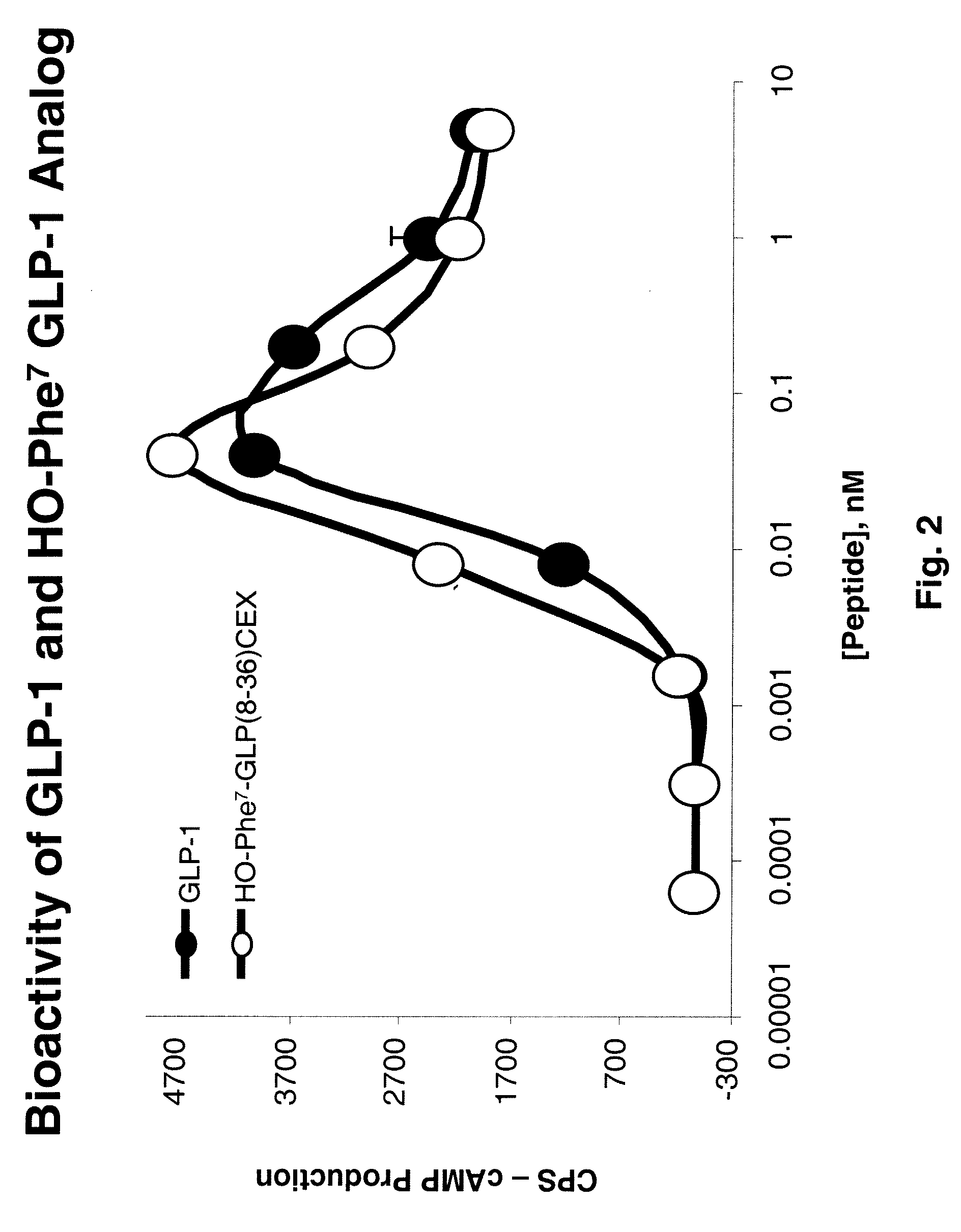
[1008]Bioassay Experimental Design: Luciferase-Based Reporter Gene Assay for cAMP Detection
[1009]The ability of each glucagon and GLP-1 analog or prodrug to induce cAMP was measured in a firefly luciferase-based reporter assay. The cAMP production that is induced is directly proportional to the glucagon or GLP-1 binding to its receptor. HEK293 cells co-transfected with the glucagon or GLP-1 receptor, respectively, and luciferase gene linked to a cAMP responsive element were employed for the bioassay.
[1010]The cells were serum-deprived by culturing 16 hours in Dulbecco Minimum Essential Medium (Invitrogen, Carlsbad, Calif.) supplemented with 0.25% Bovine Growth Serum (HyClone, Logan, Utah) and then incubated with serial dilutions of either GLP-1 analogs or prodrugs for 5 hours at 37° C., 5% CO2 in 96 well poly-D-Lysine-coated “Biocoat” plates (BD Biosciences, San Jose, Calif.). At the end of the incubation, 100 μL of LucLite luminescence substrate reagent (Perkin Elmer, Wellesley, Ma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| chemical cleavage half life | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com