Novel heparin entities and methods of use
a technology of heparin and heparin-like cells, applied in the field of new heparin-like cells, can solve the problems of adverse body response, loss of medical device function, and negative impact on the biological activity of these “biologics”
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
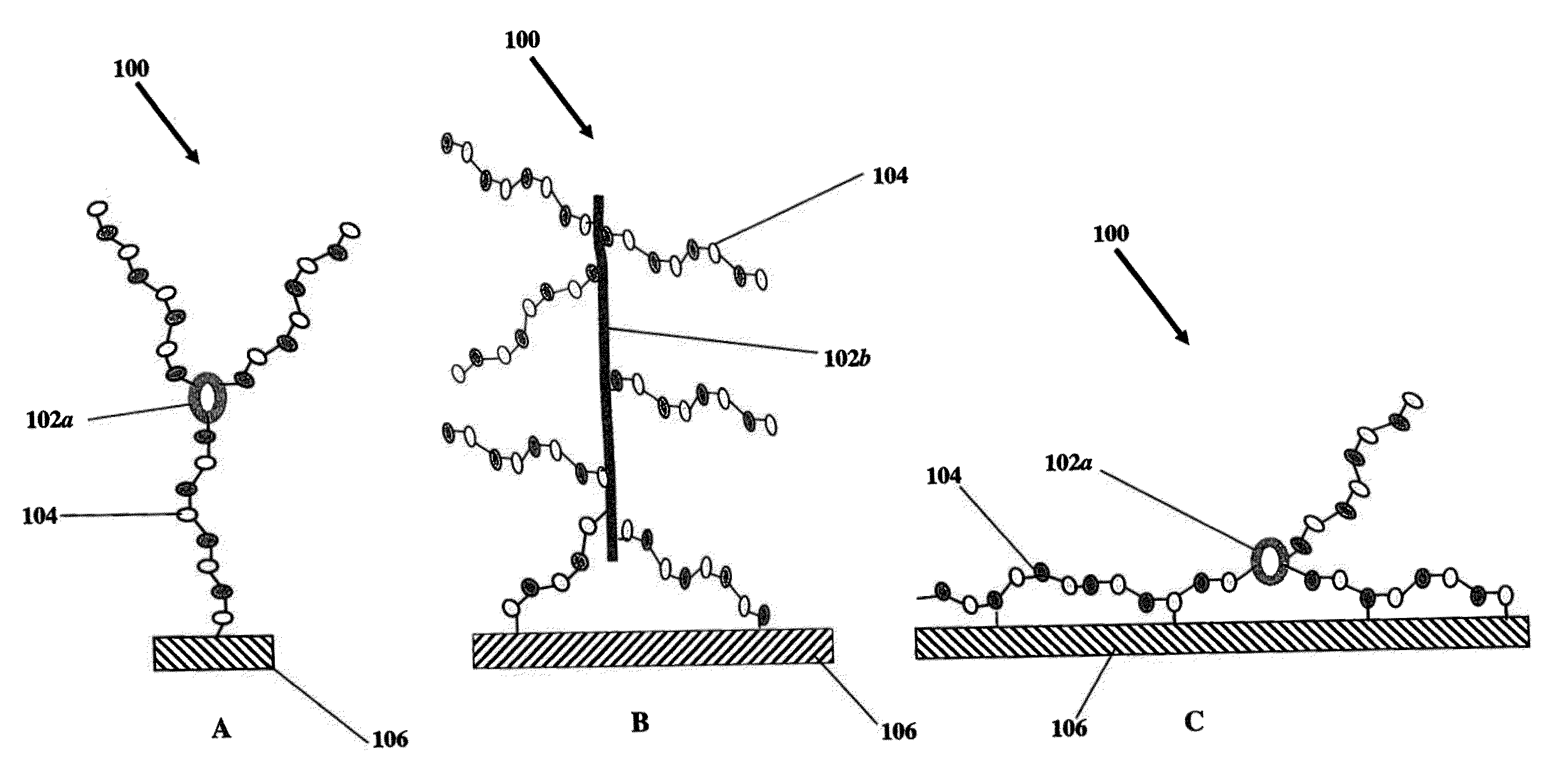
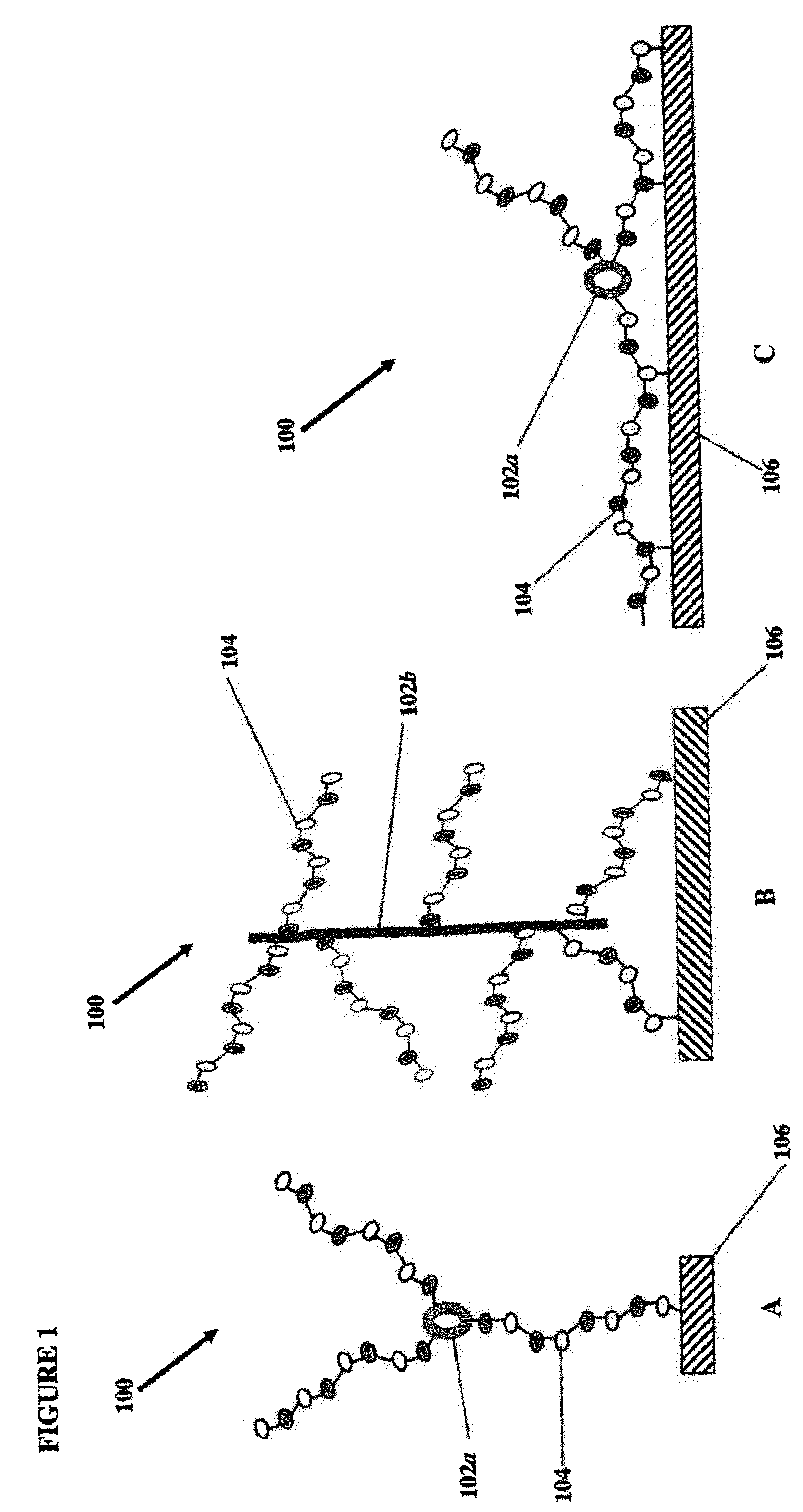
[0065]This example describes the construction of heparin entities comprising heparin and colistin sulfate as the core. This heparin entity contains free terminal aldehydes that can be used for attachment to a surface of a substrate.
[0066]Colistin sulfate (0.10 g, Alpharma, Inc.) was dissolved in 300 ml of deionized (DI) water containing MES buffer (pH 4.7, BupH™ Thermo Scientific). To this was added 10 g USP heparin, 4 g N-hydroxysulfosuccinimide (sulfo-NHS, Thermo Scientific), and 4 g of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC hydrochloride, Sigma-Aldrich, St. Louis, Mo.). The reaction was allowed to proceed at room temperature for 4 hours, followed by dialysis overnight with a 50,000 MWCO membrane (Spectra / Por®). The retentate (about 350 ml out of 500 ml) was transferred to a beaker, and cooled to 0° C. Sodium nitrite (10 mg) and acetic acid (2 ml) were added and the reaction was allowed to proceed for 1 hour at 0° C. Dialysis was performed overnight with a 50,000 MWCO ...
example 2
[0067]This example describes the construction of heparin entities comprising heparin and neomycin sulfate as the core. This heparin entity contains free terminal aldehydes that can be used for attachment to a surface of a substrate.
[0068]Neomycin sulfate (0.0646 g, Spectrum Chemical) was dissolved in 300 ml of DI water containing MES buffer (pH 4.7, BupH™ Thermo Scientific). To this was added 10 g USP heparin, 4 g N-hydroxysulfosuccinimide (sulfo-NHS), and 4 g of EDC hydrochloride. The reaction was allowed to proceed at room temperature for 4 hours, followed by dialysis overnight with a 50,000 MWCO membrane (Spectra / Por®). The retentate (about 400 ml out of 505 ml) was transferred to a beaker and cooled to 0° C. Sodium nitrite (10 mg) and acetic acid (2 ml) were added and the reaction was allowed to proceed for 1 hour at 0° C. Dialysis was performed overnight with a 50,000 MWCO membrane with the addition of 1 g NaCl to the dialysis liquid. The dialyzed retentate was filtered twice u...
example 3
[0069]This example describes the construction of heparin entities comprising heparin and capreomycin sulfate as the core. This heparin entity contains free terminal aldehydes that can be used for attachment to a surface of a substrate.
[0070]Capreomycin sulfate (0.0501 g, Sigma-Aldrich, St. Louis, Mo.) was dissolved in 300 ml of DI water containing MES buffer (pH 4.7, BupH™ Thermo Scientific). To this was added 10 g USP heparin, 4 g N-hydroxysulfosuccinimide (sulfo-NHS), and 4 g of EDC hydrochloride. The reaction was allowed to proceed at room temperature for 4 hours. The reaction mixture was filtered once using a 20 micrometer, 0.00079 inches U.S.A. standard testing sieve, A.S.T.M.E.-11 specification No. 635 to remove small particles and the filtrate was dialyzed overnight with a 50,000 MWCO membrane (Spectra / Pore). The retentate (about 400 ml out of 515 ml) was transferred to a beaker and cooled to 0° C. Sodium nitrite (10 mg) and acetic acid (2 ml) were added and the reaction was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com