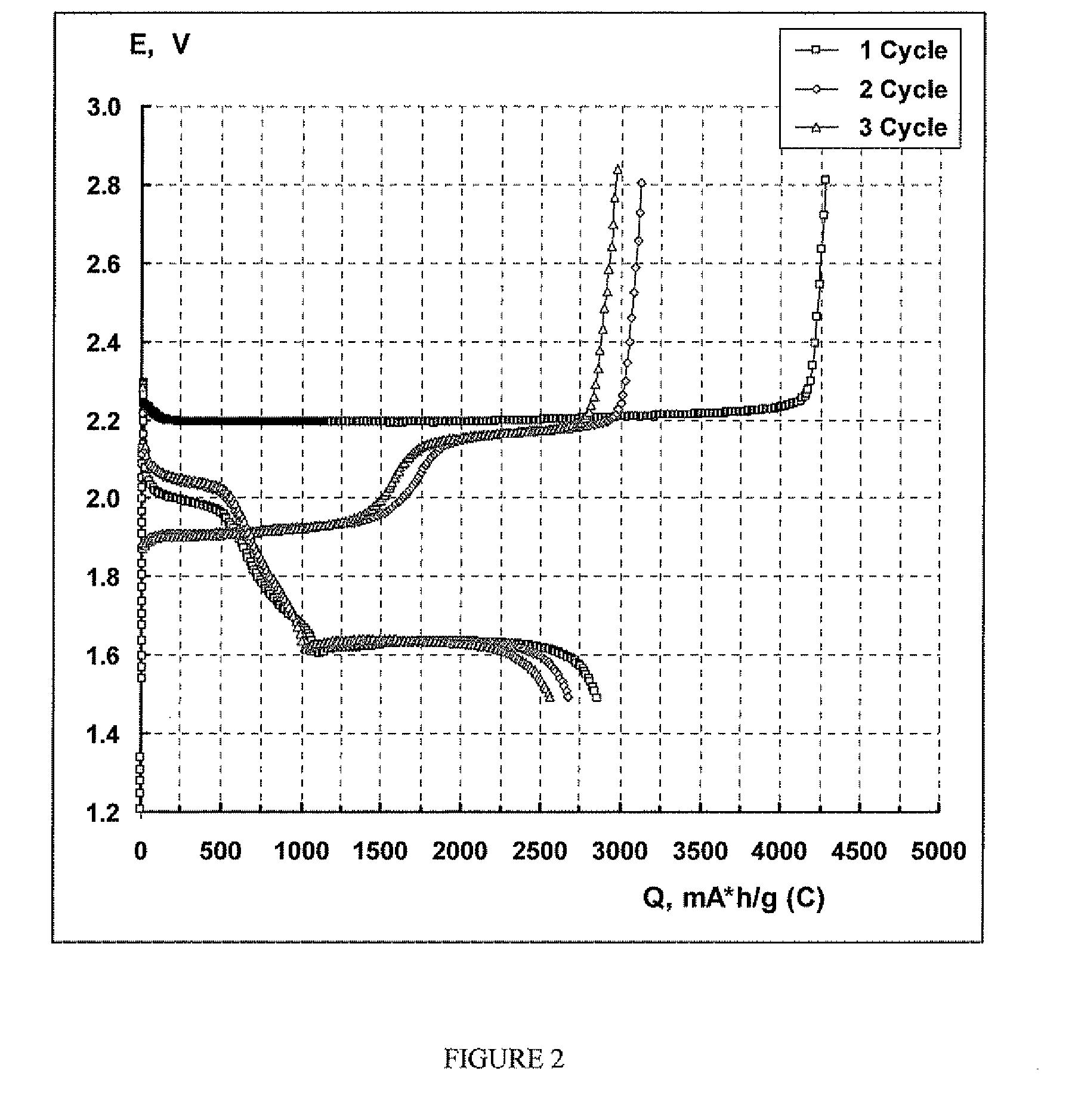
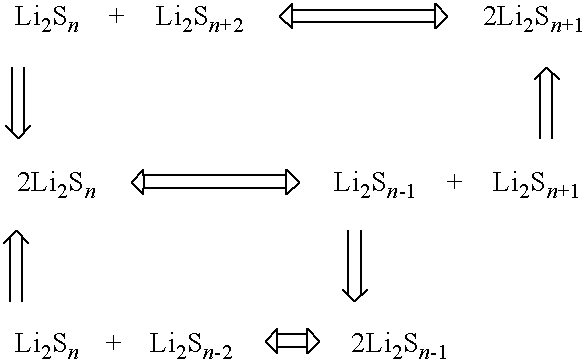Lithium sulphide battery and method of producing the same
a lithium-sulphide battery and lithium sulphide technology, applied in the field of electrochemical power engineering, can solve the problems of limiting the commercialization of lithium-sulphur batteries, moderate cycle life, and the present applicant has not been able to find specific examples of lithium-sulphur batteries in the available literature, so as to increase the cycling depth of lithium-sulphur batteries, reduce the length of lithium polysulphide chains, and improve the cycle life of lithium-sulphur batteries
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0052]Lithium sulphide, 98% (Sigma-Aldrich, UK) and sublimated sulphur, 99.5% (Fisher Scientific, UK) were ground at a mass ratio of 90:10 in a high speed mill (Microtron MB550) for 15 to 20 minutes in an atmosphere of dry argon (moisture content 20-25 ppm). The ground mixture of lithium sulphide and sulphur was placed into a flask and an electrolyte was added to the flask. A 1M solution of lithium trifluoromethanesulphonate (available from 3M Corporation, St. Paul, Minn.) in sulfolane (99.8%, standard for GC available from Sigma-Aldrich, UK) was used as the electrolyte. The liquid to solid mass ratio was 10:1. The content of the flask was mixed for 24 hours by means of a magnetic stirrer at room temperature. The liquid phase was separated from the non-dissolved solid phase by filtration. Then the sulphur in the form of sulphides and the total sulphur content were analysed. The content of the total sulphur in the initial electrolyte was also analysed and taken into account.
The Analy...
example 2
[0057]The solution of polysulphides in electrolyte was prepared as described in the Example 1 (1M solution of lithium trifluoromethanesulphonate in sulpholane) and the total amount of sulphur and sulphide was chemically analyzed. The mass ratio of Li2S:S was 50:50.
The Analysis Results:
[0058]
The total sulphur content in the initial25.8 ± 0.1electrolyte, % by massThe total sulphur content in the electrolyte31.8 ± 0.1after the reaction with the mixture of thesulphur and lithium sulphide, %The content of sulphide sulphur in electrolyte0.96 ± 0.05after the reaction with the mixture of sulphurand lithium sulphide, %
[0059]The content and the composition of lithium polysulphides in the electrolyte after the reaction of lithium sulphide with sulphur were calculated based on the analysis results.
Calculation Results:
[0060]Polysulphide composition: Li2S6,25
[0061]Concentration: 0.96%
example 3
[0062]The solution of polysulphides in electrolyte was prepared as described in the Example 1 (1M solution of lithium trifluoromethanesulphonate in sulpholane) and the amount of sulphur and sulphide sulphur was chemically analysed. The mass ratio of Li2S:S was 10:90.
The Analysis Results:
[0063]
The total sulphur content in the initial25.8 ± 0.1electrolyte, % by massThe total sulphur content in electrolyte29.9after the reaction with the mixture of sulphurand lithium sulphide, %The content of sulphide sulphur in electrolyte0.7after the reaction with the mixture of thesulphur and lithium sulphide, %
[0064]The composition of lithium polysulphides in the electrolyte after the reaction of lithium sulphide with sulphur and the concentration of lithium polysulphide in electrolyte were calculated based on the analysis results.
Calculation Results:
[0065]Polysulphide composition: Li2S5,86
[0066]Concentration: 0.7%
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific energy | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
| volume density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com