Batteries and electrodes for use thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
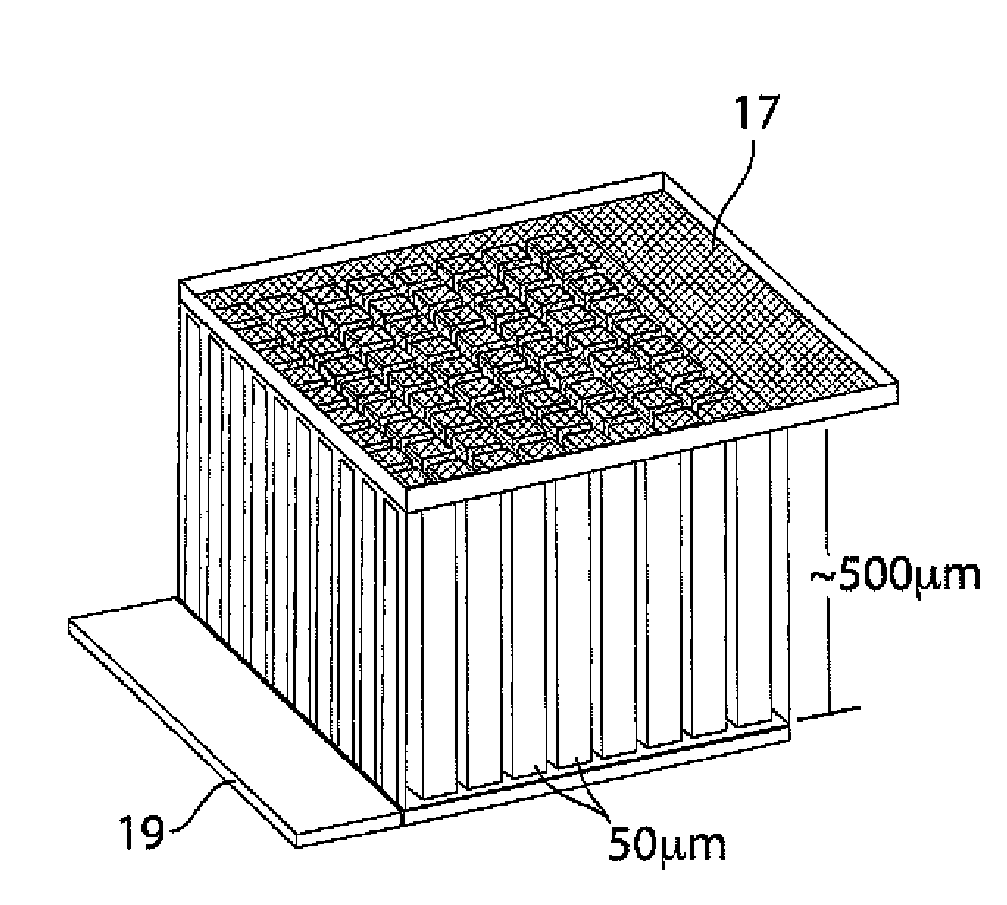
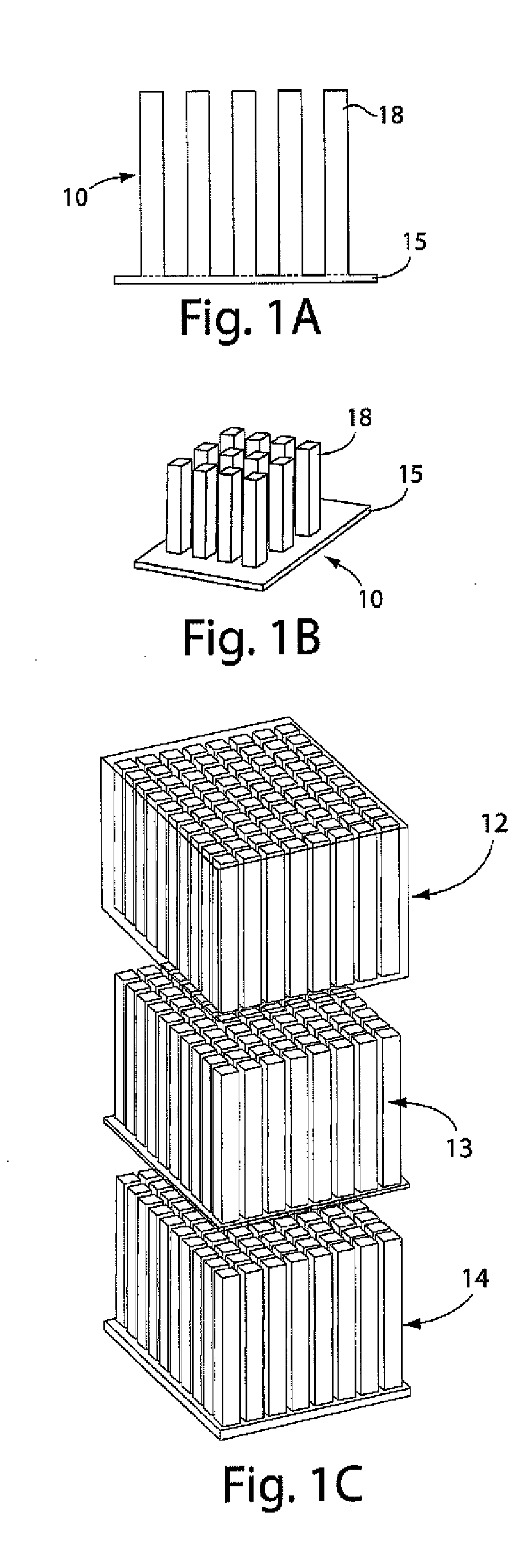
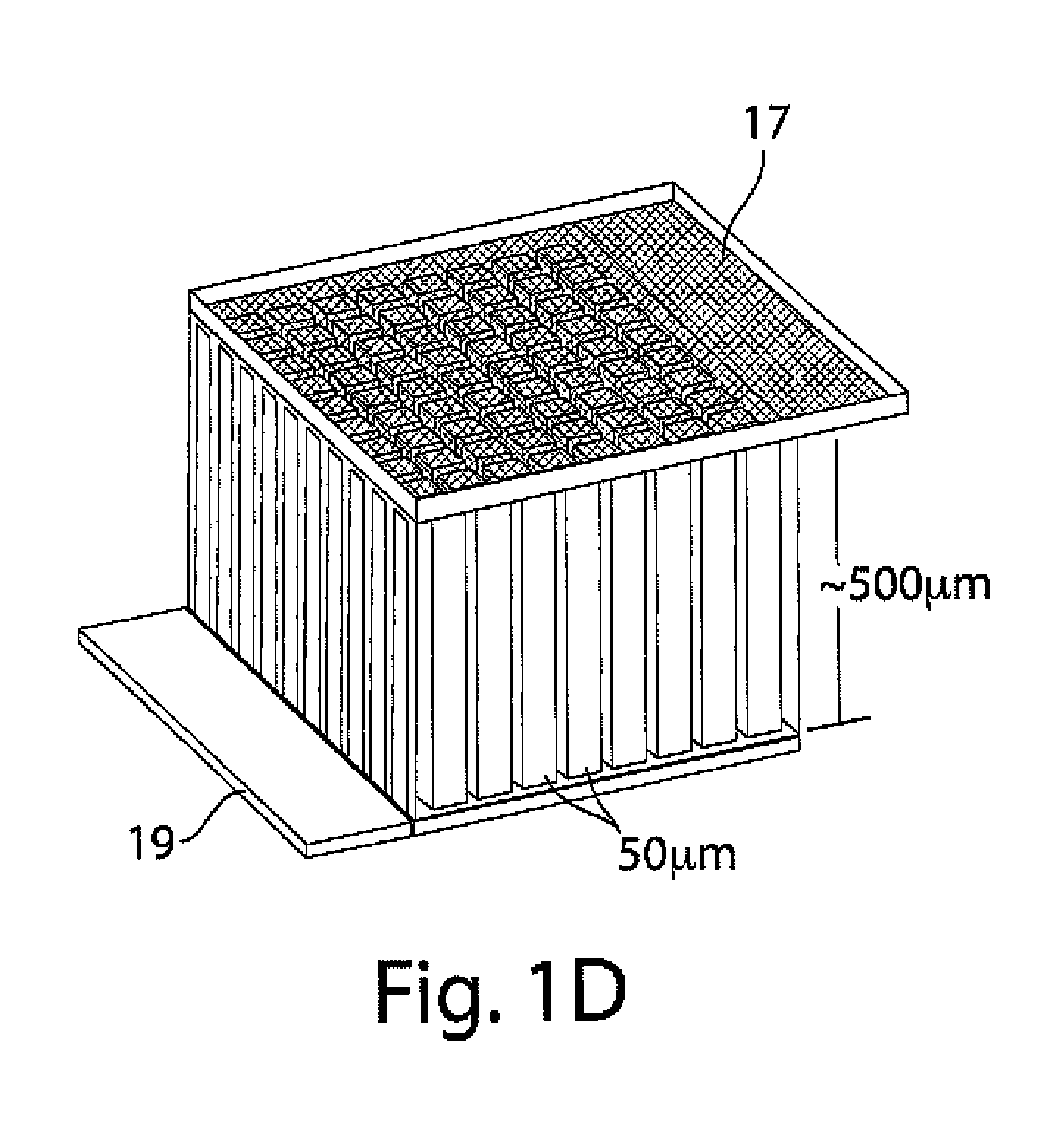
[0109]This example illustrates an integrally packaged, solid-state lithium rechargeable microbattery with a 3-dimensional interpenetrating-electrode internal architecture, in accordance with one embodiment of the invention. Such microbatteries may have the capability for outer package aspect ratios of (for example) less than 5:1 for maximum to minimum dimensions (i.e., not restricted to thin planar configurations), active materials packaging fraction of >75% in a 1 mm3 volume, under which conditions they will exceed an initial energy density target of 200 W h / l by a factor of 3 to 7. The approach in this example will use currently available and proven cathode and anode materials, but does not exclude higher energy or higher rate active materials in the future.
[0110]The microbatteries in this example will allow energy densities of about 200 W h / l to about 1500 W h / L to be achieved, depending on the electrochemical couple used, and specific design parameters, as discussed below. Micro...
example 2
[0119]In this example, 3D batteries having periodic or aperiodic interpenetrating electrodes are used since their electronic conductivity is typically higher than ionic conductivity in battery materials. Interpenetrating electrodes of high aspect ratio can have shorter ion diffusion length between electrodes while still taking advantage of the higher electronic conductivity along the electrodes to extract current. In the solid-state diffusion limit, the dimension that may determine the utilization of the battery capacity is the half-width x of the electrode features, for which the discharge time is t=x2 / DLi.
[0120]Using tabulated room-temperature lithium chemical diffusivities (DLi) for spinel and layered structure intercalation oxides, which fall in the range 1×10−9 cm2 / sec to 5×10−9 cm2 / sec, for a maximum 2C discharge rate (t=1800 sec), a half-thicknesses of about 6 to about 30 micrometers is useful. These kinetics and their limitations on particle dimensions are well-known to the ...
example 3
[0130]In this example, it is shown that a porous sintered electrode of LiCoO2 of greater than 0.5 mm minimum cross-sectional dimension that is infused with a liquid electrolyte can, surprisingly and unexpectedly, be electrochemically cycled while obtaining nearly all of the available ion storage capacity over at least 20 cycles at C / 20 rate with minimal capacity fade and no apparent detrimental mechanical damage to the electrode. This shows that such electrodes can effectively be used in certain batteries of the invention.
[0131]A battery grade LiCoO2 powder from Seimi Corporation (Japan) having 10.7 micrometers d50 particle size was pressed and fired at 1100° C. in air to form a porous sintered ceramic having about 85% of the theoretical density of LiCoO2. In one instance, a plate of this electrode having 0.66 mm thickness was prepared, as shown in FIGS. 8A and 8B. This electrode plate was attached to a gold foil current collector and assembled for testing in a sealed polymer pouch-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com