Immune Agglutination Reagent Kit and Method of Measuring Antigen
a technology of immunoagglutination and reagents, applied in the field of monoclonal antibodies, can solve the problems of insufficient amount of latex aggregates formed and inability to achieve desired absorbance, so as to improve the accuracy of measurement, reduce economic costs, and expand the range of measurement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of a Native and Natural CDT Fraction
[0047]An iron saturated solution (250 μl of 0.5M NaHCO3 and 180 μl of 10 mM FeCl3) was added to 10 mL of a high-level CDT human serum and incubated for an hour at 4° C. A delipidated solution (100 μl of 100 g / l sulfuric dextran and 500 μl of 1M CaCl2) was then added and the supernatant of the solution was collected. In addition, the albumin was removed using Blue Sepharose column (Product name: Blue Sepharose 6 Fast Flow; manufactured by GE Healthcare) followed by the removal of globulin using a Protein G column (Product name: Hi Trap Protein G HP, manufactured by GE Healthcare) to ultimately remove all proteins besides transferrin. CDT fraction containing native disialo-transferrin and asialo transferrin was prepared by using an anion-exchange column (Product name: Mono Q HR; manufactured by GE Healthcare).
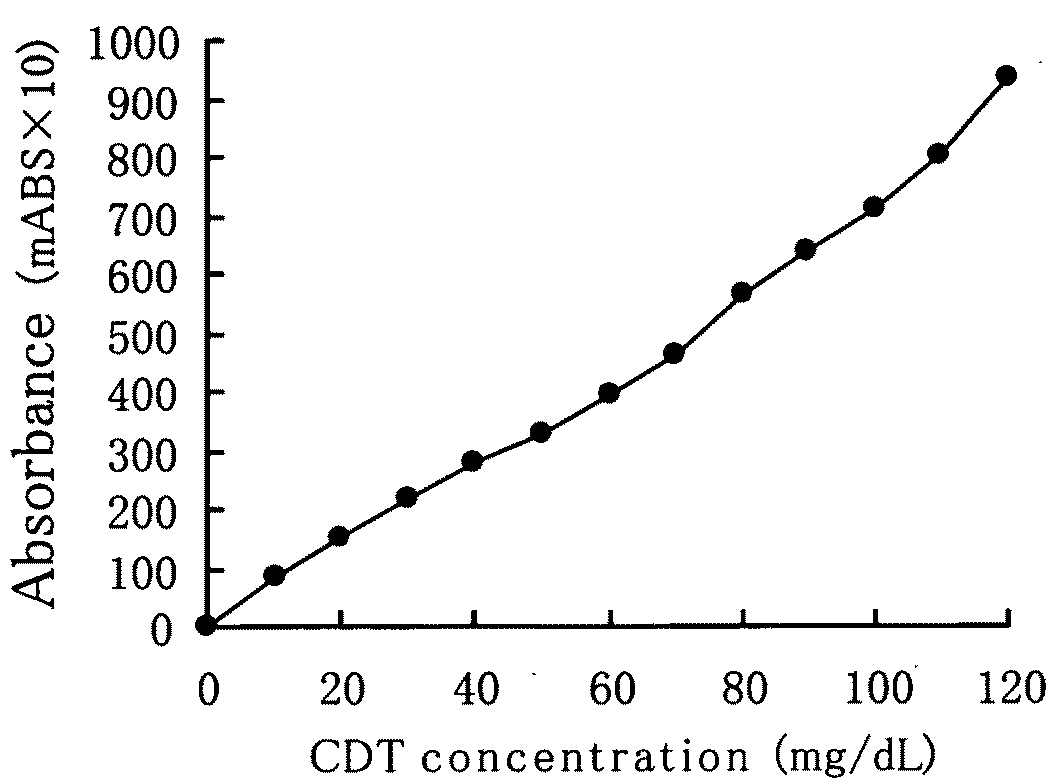
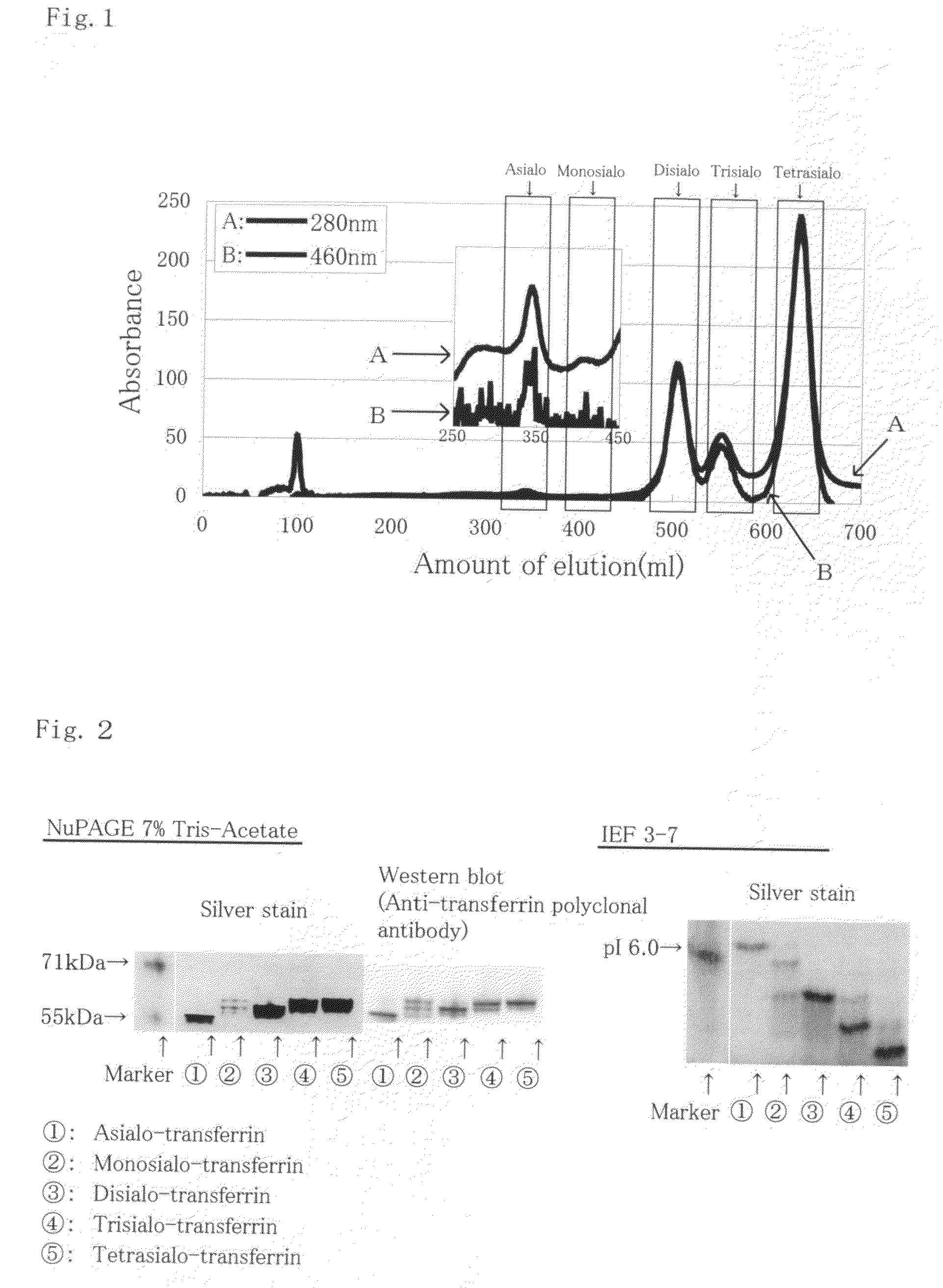
[0048]FIG. 1 shows a graph of the CDT fraction elution profile by anion-exchange chromatography. Arrow A in the Drawing shows the ...
example 2
Preparation of Hybridoma
[0049]The fraction containing disialo- and asialo-transferrin obtained in the above Example 1 was used as a CDT antigen. The antigen solution with a concentration of 0.5 mg / mL was prepared from the CDT antigen. 500 μl adjuvant (Product name: TiterMax Gold; manufactured by TiterMax Co.) was mixed in with 500 μl of antigen solution and then emulsified. This was used to subcutaneously immunize a 5-week old BALB / c mouse. Three cycles of booster using the same prepared solution was performed with 2 weeks in between cycles. During this period, a blood sample was taken at the time of immunization to measure antibody activity in the blood. 14 days after the last immunization, 100 μl of antigen solution was administered into the abdominal cavity and 3 days later the spleen was extracted. Splenic cells were made to react for 2 min. in the presence of mouse myeloma cells (P3x63Ag8.653) and polyethylene glycol 4000 (Product name; Merck Inc.) for fusion to occur after whi...
example 3
Selection of Antibody Producing Hybridoma for CDT Fraction
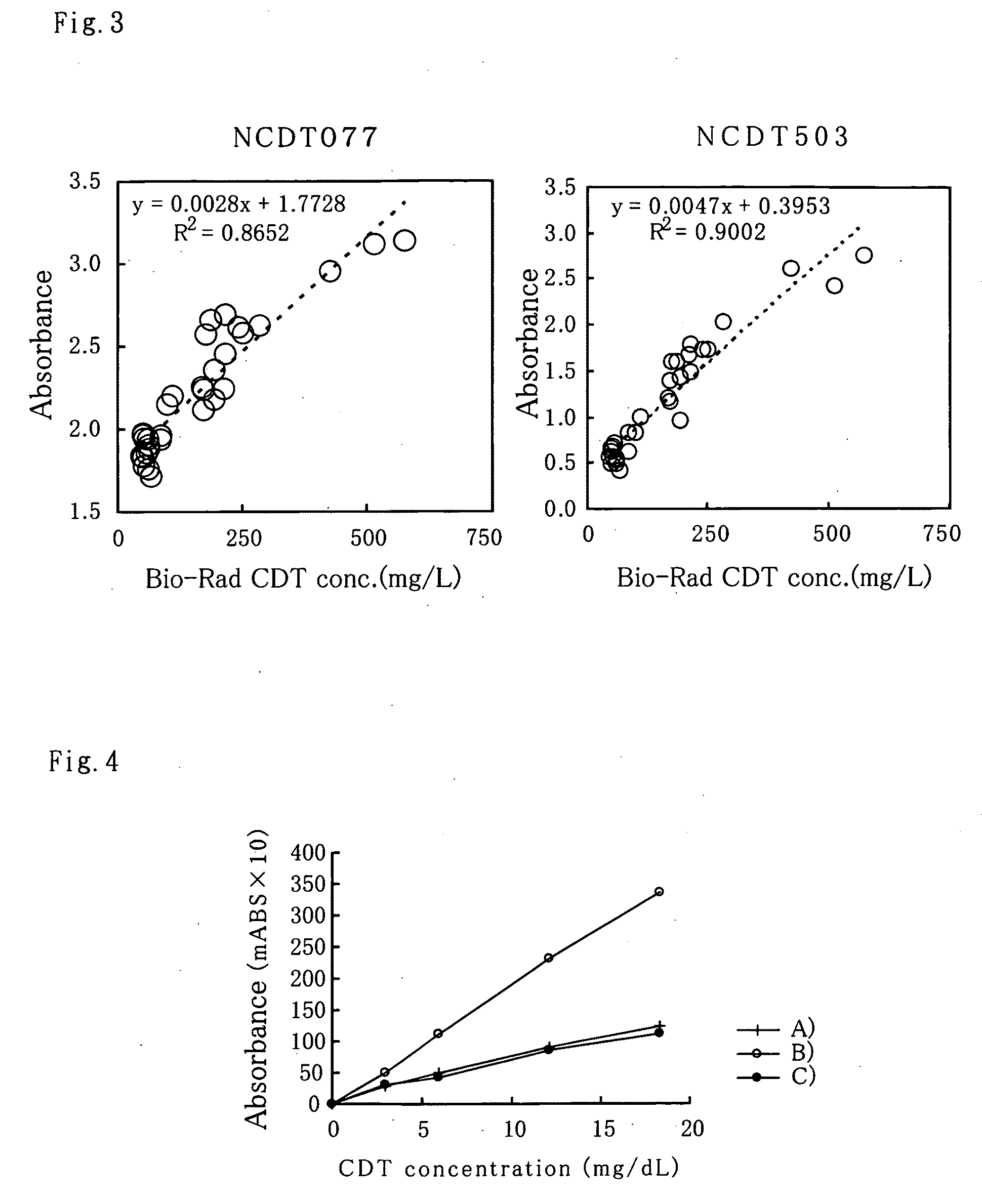
[0050]With regards to hybridoma obtained in the above Example 2, selection of antibody producing hybridoma of this invention was done by sandwich ELISA. Anti mouse antibody adjusted to a 10 μg / mL with PBS was added to a 96-well microplate, 50 μl per well, and made to react all day and night at 4° C. The wells were then washed once with PBS and blocked with 0.5% BSA-PBS to be used as the plate for screening. 50 μl of culture supernatant from wells with confirmed hybridoma growth was added to the wells and made to react for 1 hour at room temperature. After washing with wash solution (0.05% Tween-PBS), the CDT fraction mentioned above in Example 1 is made to react for 1 hour at room temperature and similarly washed. A 50 μl alkaline phosphatase labeled anti-transferrin polyclonal antibody is then added and made to react for 1 hour at room temperature. The alkaline phosphatase labeled anti-transferrin polyclonal antibody used wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com