Antimicrobial polymer conjugates
a polymer and antimicrobial technology, applied in the field of conjugates of antimicrobial agents, can solve the problems of small proteins (less than about 70 kda), significant morbidity and mortality, and high lethal death, and achieve the effects of reducing antibody binding, increasing circulating half-life, and retaining antimicrobial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Study of PEGylated Lysostaphin
Lysostaphin PEGylation
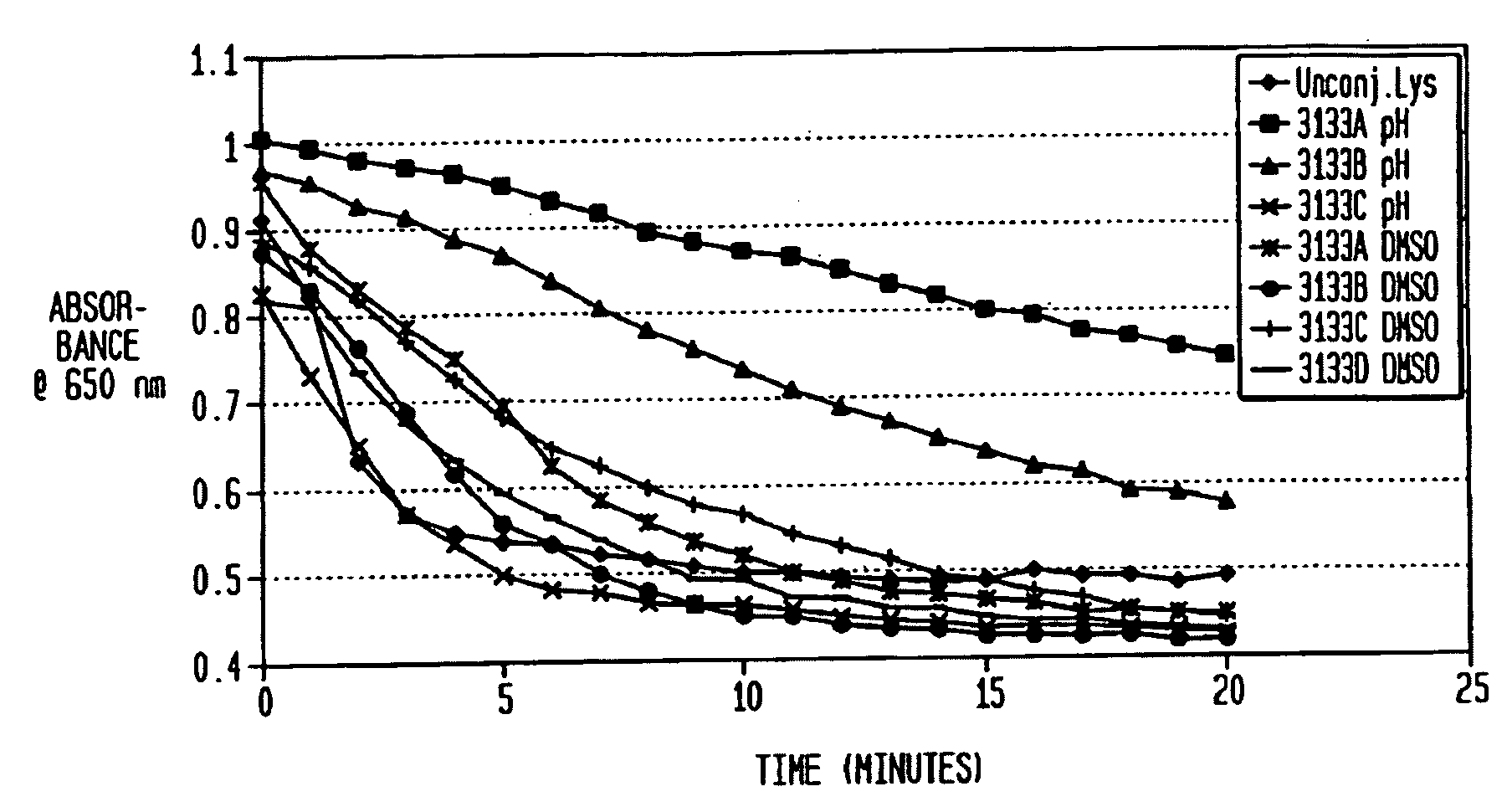
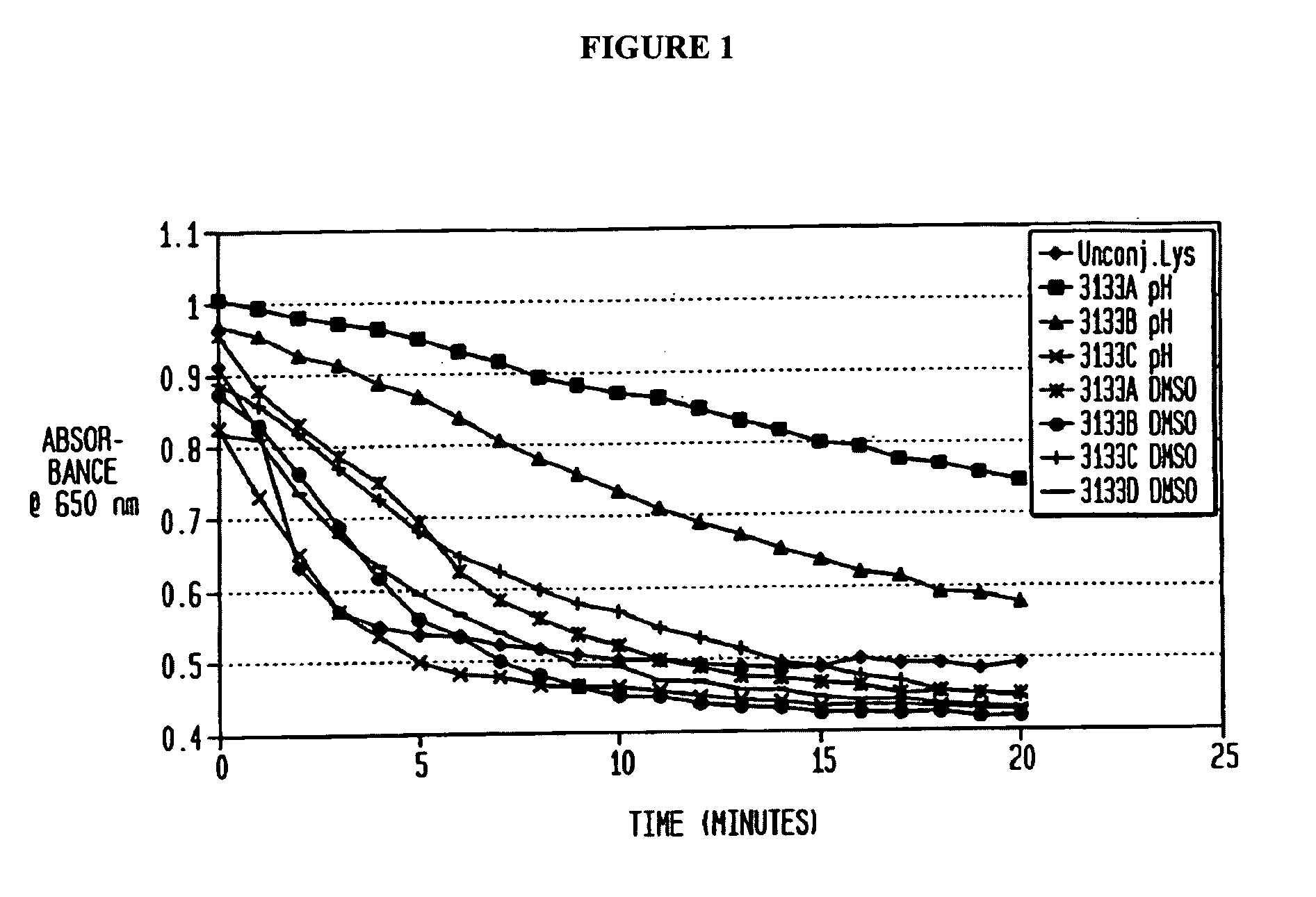
[0058]Lysostaphin at 0.27, 1, or 5 mg / mL was dissolved in either 0.2M borate buffer (pH 8.5) or DMSO. The mPEG2-NHS esters were prepared in DMSO and added to the lysostaphin solution in molar excess at ratios of 40, 20, 10, 5 or 2.5:1. PEGylation was performed with three different buffer conditions, all at room temperature for 1, 2, or 3 hours: borate buffer (with <10% DMSO contributed by adding PEG), 50% borate / 50% DMSO, and 100% DMSO. All reactions were quenched by added glycine to 25 mM and vortexing.
[0059]PEG conjugation to lysostaphin was evaluated by SDS-PAGE with the NuPage Electrophoresis System. Non-reduced samples (300 ng) were run on a Novex 4-12% Bis-Tris gel at 115V and stained with colloidal blue. PEGylated lysostaphin was separated from unreached lysostaphin by running the reaction mixture over a SEPHACRYL S-100HR column. Purified PEG-lysostaphin was concentrated and saved for activity assays.
[0060]Alternatively, unc...
example 2
Fractionation of 40 kD PEG Lysostaphin Conjugates
[0073]Fractionation of the various 40 kD PEG-lysostaphin conjugate species of Example 1 was performed by ion-exchange chromatography as a means to test enzyme activity as a function of PEG conjugation number. Although perfect resolution was not achieved, fractions tended to be enriched in just one specific band. The mono-PEGylated form was purified to greater than 99% 1-mer, while the di-PEGylated form was purified to 93% 2-mer with the remainder contributed mostly by the 1-mer, as determined by size-exclusion chromatography HPLC.
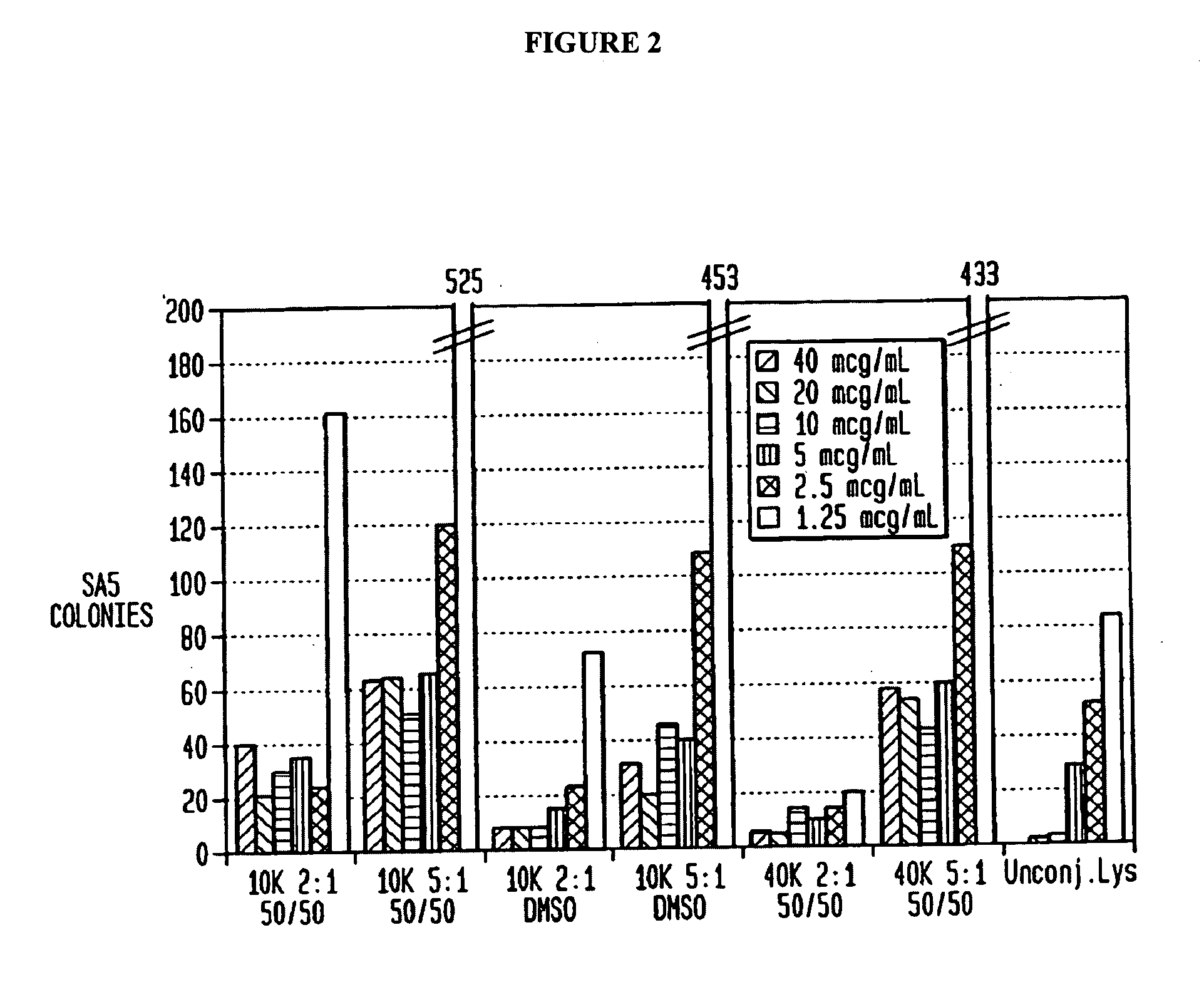
[0074]Killing Assay for Activity: The ability of lysostaphin to kill SA in saline was tested with varying concentrations of the enzyme. The bacteria were streaked onto blood agar plates after a 1-2 hour incubation with lysostaphin and surviving colonies were counted the next day. The data is reported in FIG. 5 as surviving colonies of SA so that the lower value on the graph, the more effective the killing of ...
example 3
Fractionation of 30 kD PEG-Lysostaphin Conjugates
[0079]Example 2 was repeated substituting 30 kD PEG for 40 kD PEG and 1-mers and 2-mers of mPEG 30 kD lysostaphin conjugates were isolated in separate fractions having the following properties:
[0080]OD drop assay: The OD at 280 nm of a high innoculum of S. aureus (SA, about 109 / mL) in saline is monitored over time. When bacteria are lysed, the OD drops and thus is a measure of lysostaphin activity. The faster the OD drops, the greater the enzyme activity. A typical standard takes 6-7 minutes to reach 50% of starting OD. The 1-mer has greater activity than the 2-mer, but both have significantly reduced activity compared to unconjugated lysostaphin (FIGS. 8 and 9).
[0081]Killing Assay for Activity: The ability of lysostaphin to kill SA in saline was tested with varying concentrations of the enzyme. The bacteria were streaked onto blood agar plates after a 1-2 hour incubation with lysostaphin and surviving colonies were counted the next d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com