Xylanases with enhanced thermophilicity and alkalophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
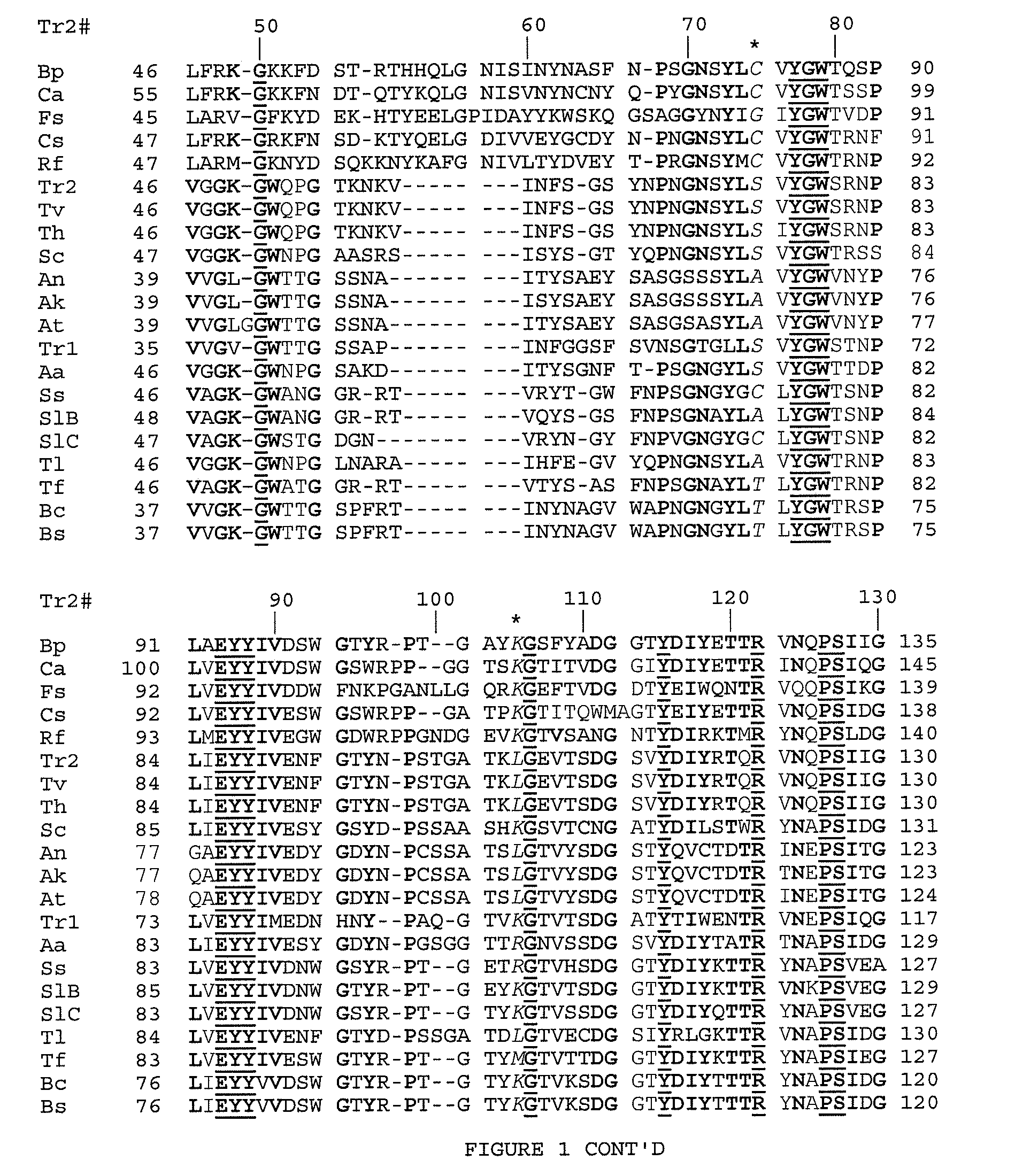
Construction of Trichoderma reesei Mutant Xylanases
[0129]Basic recombinant DNA methods like plasmid preparation, restriction enzyme digestion, polymerase chain reaction, oligonucleotide phosphorylation, ligation, transformation and DNA hybridization were performed according to well-established protocols familiar to those skilled in the art (e.g. Sung et al., 1986) or as recommended by the manufacturer of the enzymes or kit. The buffers for many enzymes have been supplied as part of a kit or made according to the manufacturer's instructions. Restriction enzymes, T4 polynucleotide kinase and T4 DNA ligase were purchased from New England Biolabs Lid, Mississauga, Ont. GeneAmp PCR reagent kit was purchased from Perkin-Elmer. A precursor plasmid pXYbc, which is a pUC type plasmid with a Bacillus circulans xylanase gene inserted, has previously been prepared and published (Sung et al, 1993; Campbell et al., U.S. Pat. No. 5,405,769). A commonly used E. coli strain, HB101 (Clonetech Lab, Pa...
example 2
Characterization of Mutant Xylanases
2-1: Production of Xylanases
[0196]The culture conditions comprised a 5 ml culture of overnight innoculant in 2YT medium (16 g bacto-tryptone, 10 g yeast extract, 5 g NaCl, 1 L of water) containing ampicillin (100 mg / L) was added to 2YT medium (1 L) with ampicillin. The cultures were grown with shaking (200 rpm) at 37° C. After 16 hr, cells were harvested.
2-2: Purification of Mutant Xylanases
[0197]Protein samples were prepared from cells by first making an extract of the cells by grinding 10 g of the cell paste with 25 g of alumina powder. After grinding to smooth mixture, small amounts (5 mL) of ice cold buffer A (10 mM sodium acetate, pH 5.5 for BcX mutants) or buffer B (10 mM sodium acetate, pH 4.6 for TX mutants) were added and the mixture ground vigorously between additions. The alumina and cell debris were removed by centrifugation of the mixture at 8000×g for 30 min.
[0198]Prior to column chromatography, the supernatant was adjusted to pH 4.6...
example 3
Thermophilicity of Mutant Xylanases
[0206]Thermophilicity was examined to test the effect of different temperatures on the enzymatic hydrolysis of soluble xylan by different mutant xylanases.
[0207]The assay procedure was similar to the standard assay with changes in the incubation temperature and time. The xylanases (15 μg / mL) and soluble birchwood xylan substrate, in 50 mM sodium citrate buffer of pH 5.5, were mixed and incubated in a circulating water bath at different temperatures. After a 30-min incubation, the amount of reducing sugars released from xylan was determined by HBAH analysis and was calculated as a relative activity, with the value at 40° C. representing 100%.
[0208]The effect of temperature on the hydrolysis of xylan by TrX-HML-75A105H-125A129E-144R (TrX-HML-AHAE-R) is shown in FIG. 3. Compared to the precursor without the H144R mutation (TrX-HML-AHAE), this mutant xylanase showed a moderately improved enzymatic activity at higher temperature. These results suggest t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com