High Temperature Amorphous Composition Based on Aluminum Phosphate
a technology of aluminum phosphate and composition, which is applied in the direction of anti-corrosion paints, metallic material coating processes, inorganic chemistry, etc., can solve the problems of unsuitable high-temperature ceramic materials, no known synthetic oxides which can remain amorphous and metastable at temperatures up to 1400° c. or greater, and aluminum phosphate is unsuitable for high-temperature ceramic materials. achieve the effect of enhancing the resulting amorphous
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
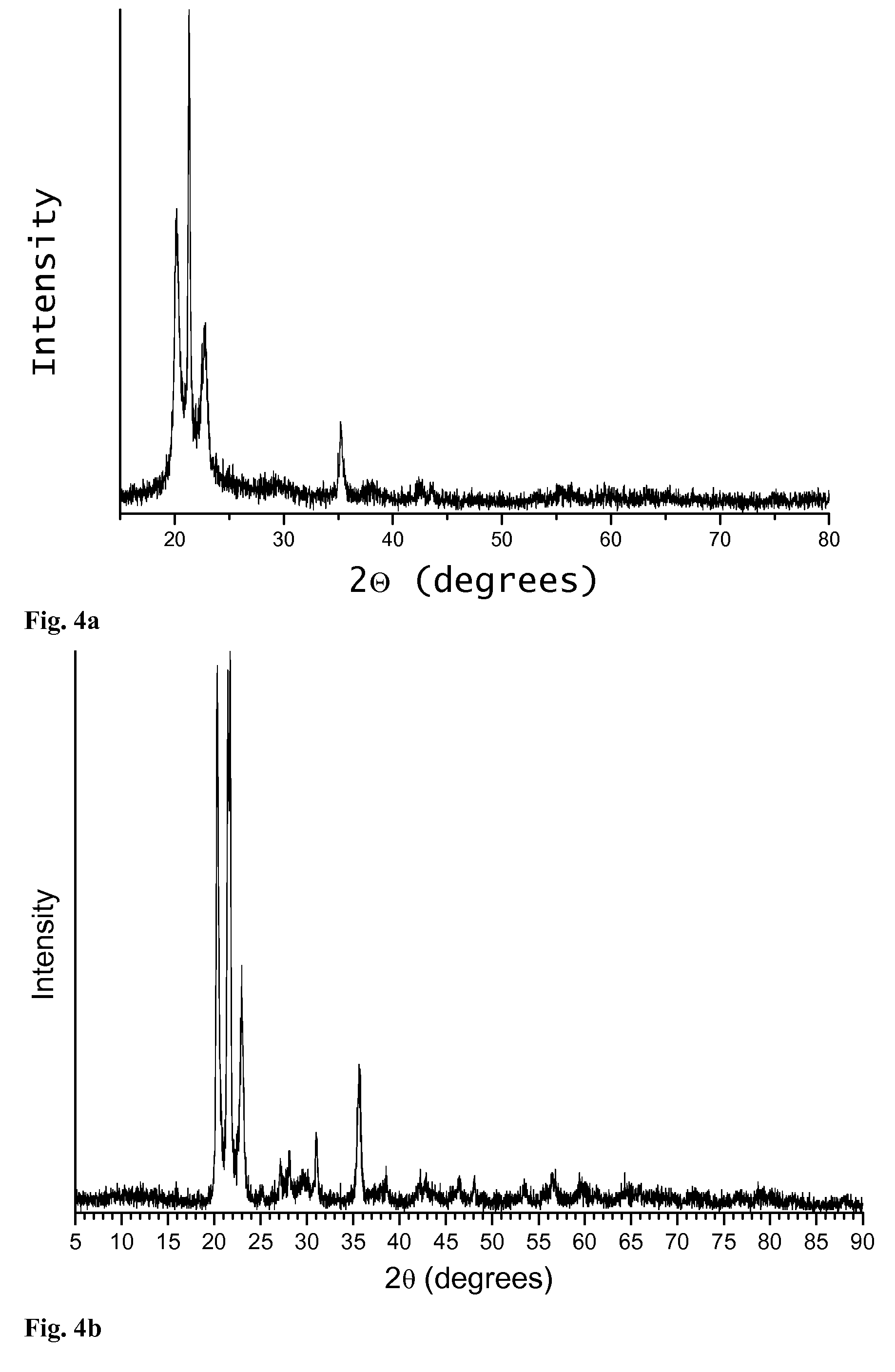
[0054]To make 850 mL of 75.46 g / L a precursor solution to synthesize the amorphous aluminum phosphate material with a 1.75:1 Al:P ratio (0.375 molar excess Al2O3), 408.94 g Al(NO3)39H2O was dissolved in 382 ml ethanol to make 500 ml of solution. In a separate container in an inert atmosphere, 25.23 g P2O5 was dissolved in 300 ml ethanol. After the P2O5 is dissolved, the two solutions were mixed together and allowed to stir for several minutes. After the solution was thoroughly mixed, it was placed in a large container in an oven at 150° C. for one or more hours. After the resulting powder is completely dried, it was annealed in air to 1100° C. for one hour to form amorphous aluminum phosphate powder with 0.75 moles excess aluminum per mole aluminum phosphate.
example 2
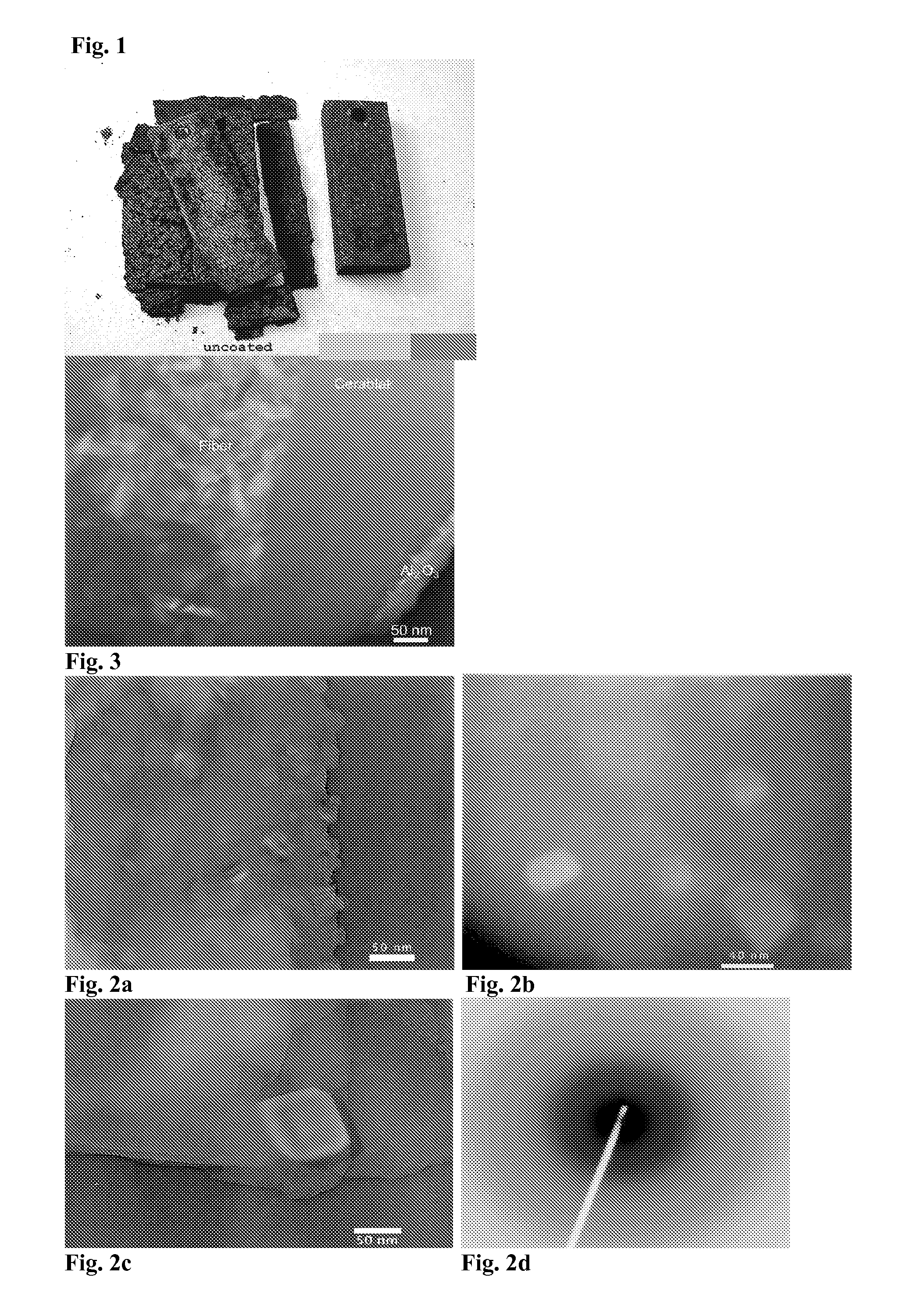
[0055]To form an oxidation resistant amorphous aluminum phosphate coating on a rectangular coupon of 304 stainless steel, the piece was dipped in the precursor solution of Example 1, diluted to a certain concentration and removed. The sample was dried in flowing air to remove the solvent. The sample was dried more thoroughly in an oven at 65° C. The piece was annealed in air to 1000° C. (at a ramp rate of 10 C / minute) for 100 hours and cooled to room temperature at 10 C / minute, along with an uncoated piece of 304 stainless steel of the same size and shape. The weight of each uncoated piece was measured prior to anneal. The weight was measured again after coating and anneal. The amorphous aluminum phosphate coated piece showed remarkably less weight gain. The weight gain data is given in the table below.
TABLE IWeight gain of uncoated, and coated (AlPO4, with 75% excess Al) stain-less steel coupons annealed to 1000° C. in air. The weight gain isrelated to the weight of the annealed, u...
example 3
[0056]To form an amorphous aluminum phosphate coating on a solid substrate by plasma spray, amorphous aluminum phosphate powder made in Example 1 is milled to a small and uniform size (around 20 microns) in a ball mill. The powder is then deposited using the small particle plasma spray process (see, U.S. Pat. No. 5,744,777 incorporated herein by reference in its entirety).
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com