Chemokine receptor antagonists as therapeutic agents
a technology of chemokine receptor and antagonist, which is applied in the field of chemokine receptor antagonists as therapeutic agents, can solve the problems of remain elusive, and the molecular mechanism used by immature dcs or other putative tolerogenic apcs to suppress t cell responses is unclear, so as to reduce immune tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
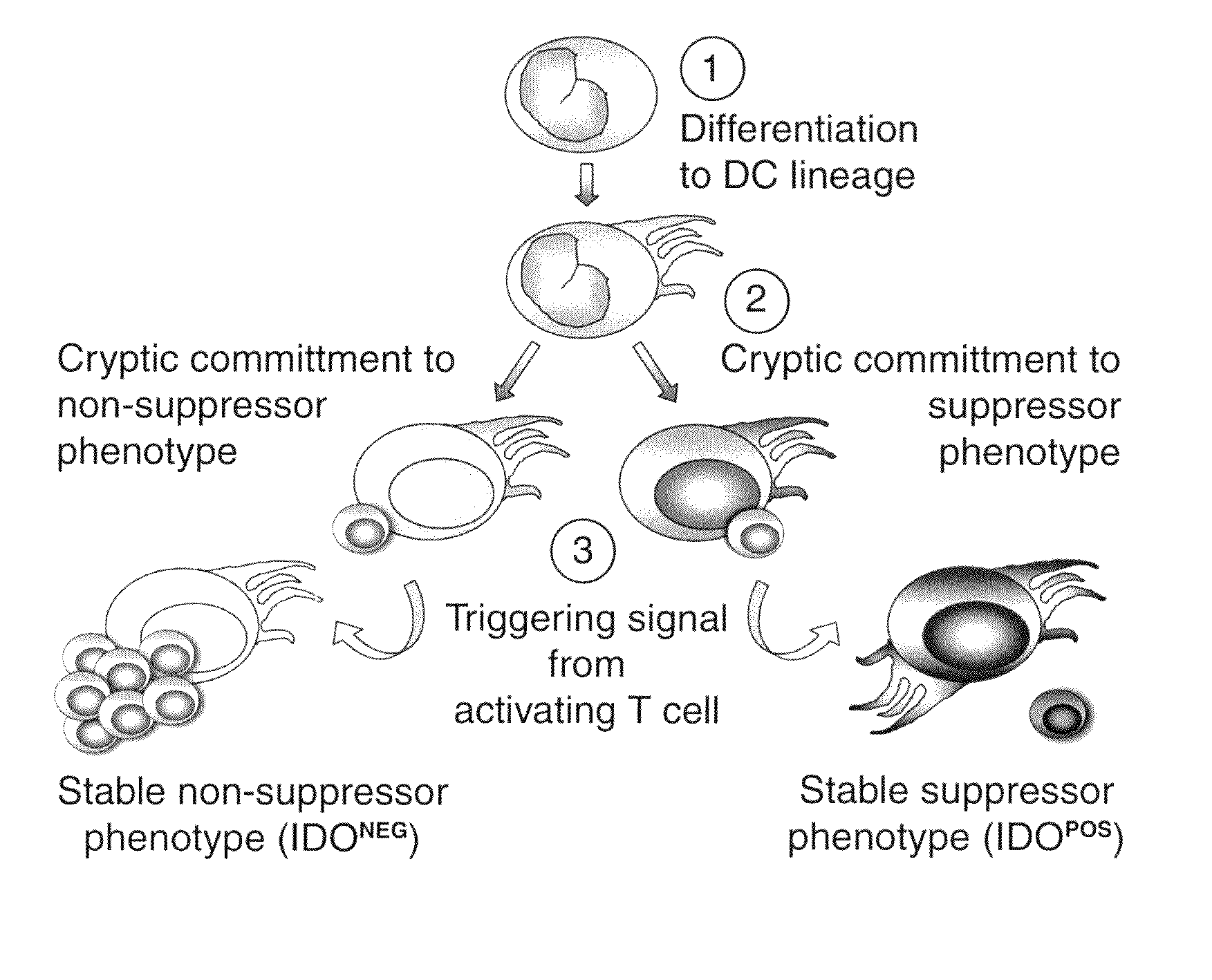
Image
Examples
example 1
Cell Culture
[0163]Human monocytes and lymphocytes were isolated by leukocytapheresis and counterflow elutriation (D. H. Munn et al., J. Exp. Med. 189, 1363-1372 (1999)). Monocytes (typically >95% purity) were cultured in 100 mm tissue culture petri dishes in RPMI-1640 medium with 10% newborn calf serum (Hyclone) and including penicillin / streptomycin and glutamine. Cultures received either MCSF (200 U / ml, Genetics Institute) on day 0, or GMCSF (50 ng / ml, R&D Systems)+IL4 (50 ng / ml, R&D Systems) on days 0, 2 and 4. For experiments where CCR6 expression was of interest, cultures received a single dose of GMCSF+IL4 (100 ng / ml each) on day 0, with no further supplementation. Loosely adherent dendritic cells (GMCSF+IL4) were harvested by gentle aspiration; adherent macrophages (MCSF) and non-dendritic APCs (GMCSF+IL4) were harvested with EDTA. Other cultures were conducted in serum-free medium (X-vivo 15; BioWhitaker, Walkersville, Md.) plus cytokines.
example 2
Production of Antibodies
[0164]All antibodies were obtained commercially except for polyclonal antiserum against human IDO which was manufactured as a work for hire by ZCB Inc., Hopkinton, Mass. All commercial antibodies and reagents were from BD Biosciences-Pharmingen (San Jose, Calif.) unless specified otherwise. For detection of cell surface antigens, DCs were triple-stained with anti-CD123-biotin (clone 7G3; it was found that clone 9F5 gave suboptimal results with dendritic cells) followed by streptavidin-perCP, plus anti-CD11c-allophycocyanin (clone S—HCL-3) or anti-CCR6-fluorescein (clone 53103.111, R&D systems, Minneapolis, Minn.). CCR6 results were also confirmed using a second anti-CCR6 antibody (clone 11A9; Pharmingen). For detection of IDO, cells were fixed and permeablized (Cytofix / Cytoperm), and then stained with rabbit anti-IDO antibody prepared against the peptide followed by polyerythrin-labeled anti-rabbit secondary antibody (Jackson Immunoresearch, West Grove Pa.) c...
example 3
Co-Expression of IDO with Cell Surface Markers CCR6, CD123, and CC11c in APCs
[0166]Expression of IDO in immature monocyte-derived (myeloid) dendritic cells (Dhodapkar, M. V., et al., J. Exp. Med. 193: 233-238 (2001)) and in immunosuppressive monocyte-derived macrophages (Munn, D. H., et al., J. Exp. Med. 189: 1363-1372 (1999)) was analyzed. FIG. 3 shows the expression of IDO and CCR6 by myeloid antigen-presenting cells which express the cell surface antigen CD123 (CD123+). Human monocytes were cultured as described above (Example 1) for 7 days with GMCSF+IL4 to produce myeloid dendritic cells (FIGS. 3A and 3C), or for 7 days in MCSF to produce macrophages (FIG. 3B) (Munn, D. H., et al., J. Exp. Med. 189: 1363-1372 (1999)). Prior to analysis, cells were treated with interferon-γ (INFγ) for 18 hrs to induce maximal expression of IDO. Harvested cells were triple-stained for CD123, CD11c and IDO. For FIG. 3D, cells were cultured as in Example 1 except in a commercial, FDA-approved serum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| color | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com