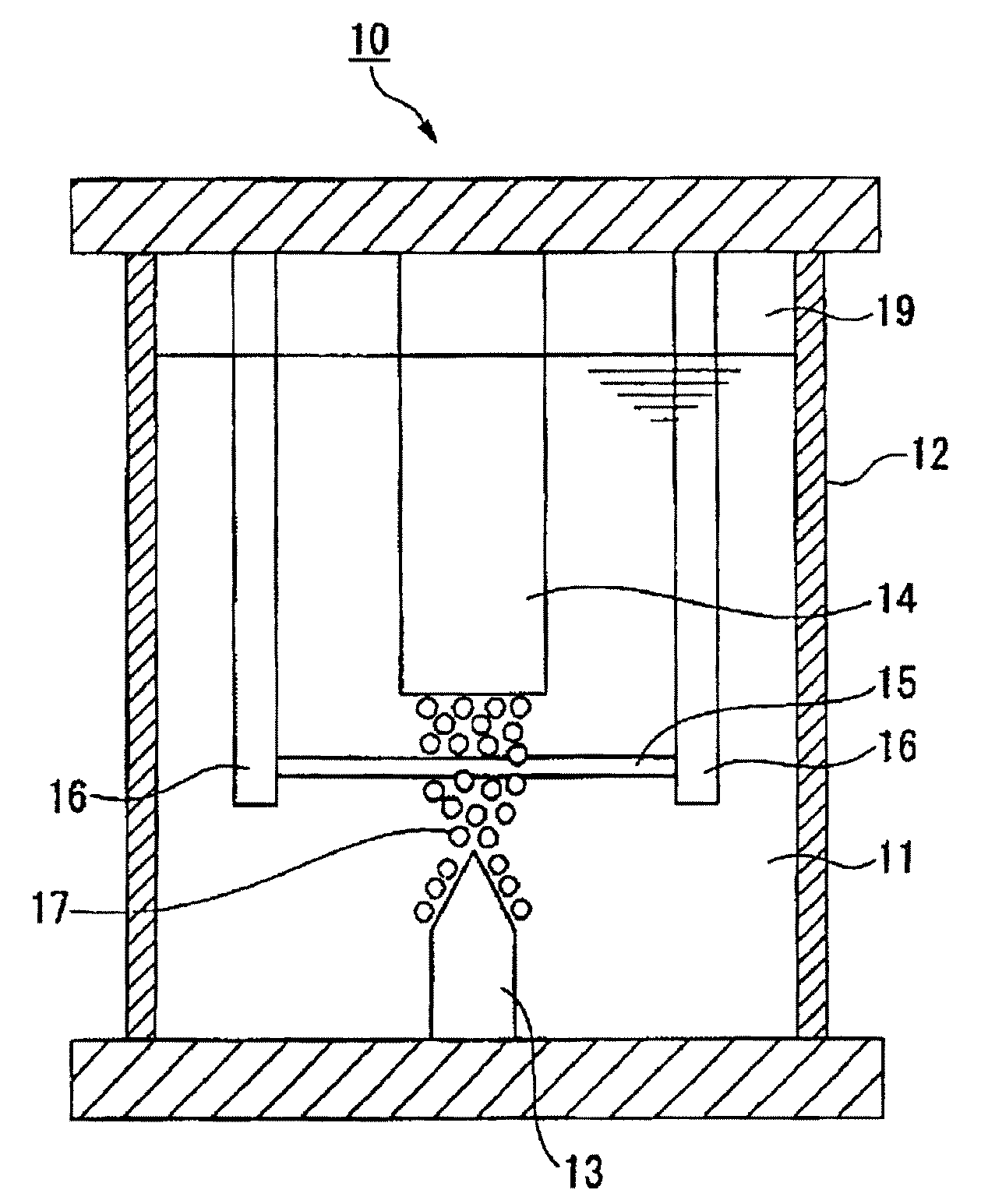
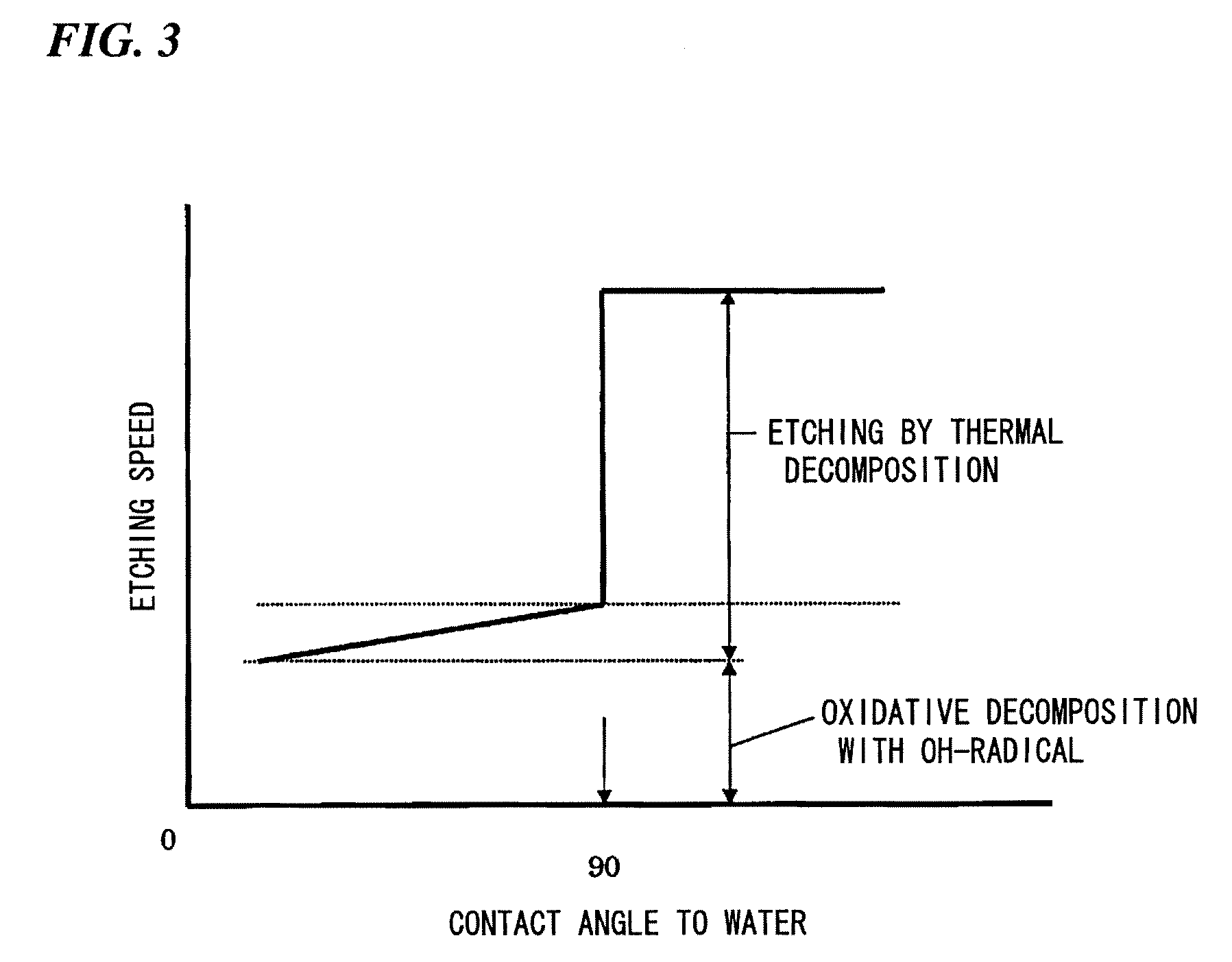
Method of Surface Treatment and Surface-Treated Article
a surface treatment and article technology, applied in the field of surface treatment of articles, can solve the problems of difficult application difficult to effective decomposition, difficult to apply to a polymeric organic material, etc., and achieve the effect of suppressing the damage of the article and reducing the damag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0122]A water-purifying device for home use (trade name: Cleansui 02) manufactured by Mitsubishi Rayon Co., Ltd. was prepared. In a filtration cartridge of the water-purifying device, a hollow fiber membrane (the contact angel of water of a raw material which is treated so as to be a hydrophilic one (25° C.)=55°) made of polyethylene which was treated so as to be a hydrophilic one was used. The hollow fiber membrane is a hollow fiber membrane having inner diameter of 270 μm and thickness of the membrane of 55 μm manufactured by Mitsubishi Rayon Co., Ltd. In the hollow fiber membrane, fibrils made of polyethylene oriented in the direction of the fiber of the hollow fiber membrane, the hollow fiber membrane had a pore structure being a slit form in which many fibrils were piled up in the direction of the thickness of the membrane.
[0123]When the water-purifying device was set to a faucet of water supply in home, and water from water supply (Mikasa-cho, Iwakuni-shi, Yamaguchi-ken, Japan...
example 2
[0129]The same plasma generating device as Example 1 was used. The sample for experiment was soaked in a container filled with water and the samples were fixed with supports in the vicinity of the electrode. Except using an ethylene / vinyl alcohol copolymer film (hereinafter, may be referred to EVAL film) shown in Table 9 as a sample, water vapor bubble plasma was generated and the plasma was contacted to the sample for 3 minutes at the same condition as Example 1. Each film had the contact angle to water before contacting to plasma being within 64 to 71° and showed hydrophilicity. These films stood the heat of water vapor bubble plasma and maintained their original forms.
TABLE 9Plasma durability6)(Gas phase of reactiondevice: atmosphericpressure)7)Measurement of contact angle of EVAL filmHaving durability8):EVAL filmgoodEthylene1)θ / 22)θ3)RH5)Not havingSample(mol %)(degree)(degree)T4) (° C.)(% RH)durability9): not goodD29082932.264.425.437GoodDC3203F3233.667.225.538GoodET38033834.669...
example 3
[0132]Nafion® 112 and Nafion® 1035 membranes manufactured by DuPont were soaked in ion-exchanged water at 25° C. for 5 minutes to swell the membranes, and the swollen membranes were taken out and the contact angles thereof were measured. The contact angles were shown in Table 10. Nafion® 112 and Nafion® 1035 shown in Table 10 were used as samples for plasma treatment.
[0133]The same plasma generating device as Example 1 was used. The samples were soaked in a container filled with water and the samples were fixed with supports in the vicinity of the electrode. Subsequently, water vapor bubble plasma was generated at the same conditions as Example 1, and the plasma was contacted to the samples for 3 minutes. As shown in Table 10, both swollen Nafion® 112 and Nafion® 1035 membranes stood the heat of the water vapor bubble plasma, and their original forms were maintained.
TABLE 10Plasma durability(Gas phase of reactiondevice: atmosphericpressure)Having durability: goodθ / 2θTRHNot having du...
PUM
| Property | Measurement | Unit |
|---|---|---|
| contact angle | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com