Dimeric Double Metal Salts of (-) Hydroxycitric Acid, Methods of Making and Uses of Same
a technology of hydroxycitric acid and dimeric double metal salts, which is applied in the field of dimeric double metal salt compositions of ()hydroxycitric acid, can solve the problems of limited therapeutic use of hca salts, and achieve the effects of reducing blood lipids, reducing blood lipids and postprandial lipemia, and increasing plasma hca levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of K / Mg-DDM-HCA and K / Mg-HCA Monomer
A. Preparation of K / Mg-DDM-HCA
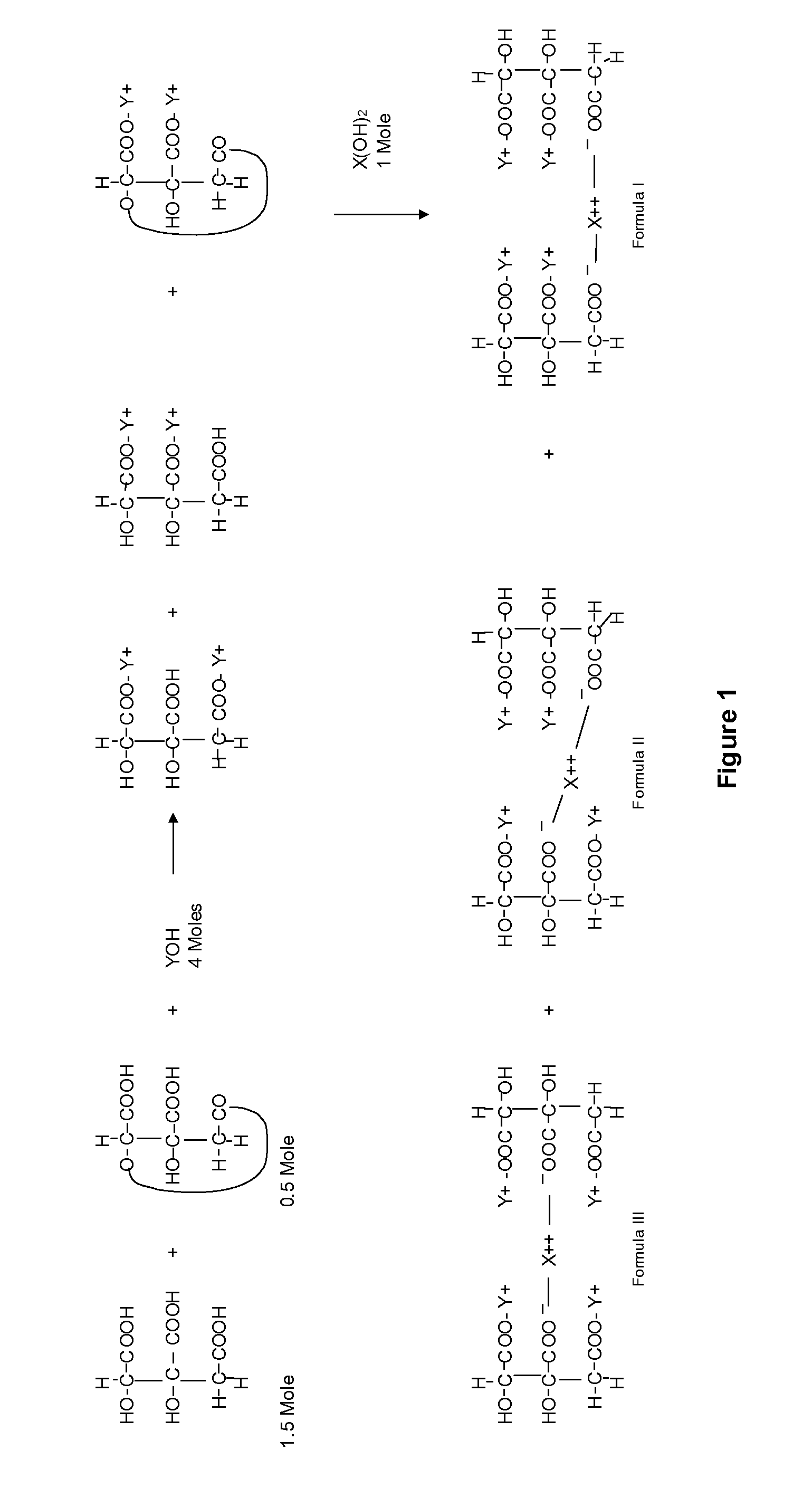
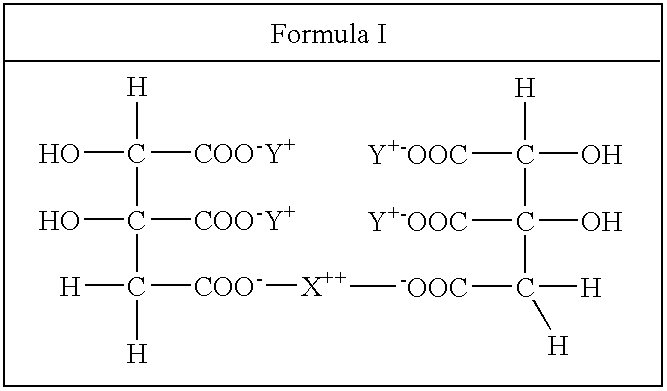
[0070]K / Mg-DDM-HCA was prepared as a mixture of DDM-HCAs of general Formula I Formula II; and Formula III, as depicted in Table 1 and described above. These structures are further detailed as K / Mg-DDM-HCAs of general Formulas V-VII, supra.
[0071]The K / Mg-DDM-HCA was prepared from a concentrated aqueous extract of the rind of the fruit of a plant of the genus Garcinia (i.e., Garcinia rind) that contains (−)-hydroxycitrate as the free acid as well as HCA lactone, i.e., HCA / lactone concentrate mixture. Specifically, the HCA was extracted from dried Garcinia rind in multiple cycles with DM water in an extractor (HR Engineering, Bangalore, India). Specifically, 600 kilograms of dried Garcinia rind was extracted in an extractor with 1200 liters of distilled water. The Garcinia rind was extracted for six (6) hours to yield a first Garcinia extract and a once-extracted Garcinia rind. The once-extracted Garcinia rin...
example 2
Comparison of Oral HCA Compositions Regarding Serum Plasma Bioavailability in a Rabbit Study
[0078]An independent study conducted by a third party, using a validated method for quantification of HCA (developed by Balint Analytical Laboratory, Budapest, Hungary) compared the levels of HCA in the serum of New Zealand white rabbits in response to oral gavage with two HCA preparations at a dose of 50 mg / kg body weight: (1) a preparation of the inventive DDM-HCA composition (67.31% HCA (KMg 24.33% / 4.02%) and (2) Super Citrimax™ containing potassium-calcium HCA (Interhealth). Thirty minutes after receiving the respective preparations, the group of three rabbits receiving the inventive DDM-HCA preparation had a mean serum HCA level of 11.3 μg / mL, a value 45.8% higher than the 6.13 μg / mL serum value of the group of three rabbits receiving the Super Citrimax™ preparation. These results demonstrate a superior bioavailability of HCA when it is delivered orally in the from of the inventive DDM-H...
example 3
Testing DDM-HCA in a Rat Model
[0079]An OM rat model is useful to test the biological properties of the DDM-HCA dosage unit forms of the invention. Briefly, male OM rats aged 10 weeks are fed a diet in which 30% of the calories are obtained from fat under standard conditions. Groups of 5-10 rats are intubated twice daily for 60 days with DDM-HCA dosage unit forms (e.g., 0.01 mmoles / kg body weight to 1 mole / kg body weight equivalent) or vehicle-control solutions with no added DDM-HCA. Blood is withdrawn from the tail vein one or more times daily. The pharmacokinetics of HCA-containing dosage unit form, including absorption, is determined by measuring the HCA level in the blood of subjects administered the HCA-containing dosage unit form using gas chromatography / mass spectroscopy (Loe et al., Anal Biochem. 2001,1; 292(1): 148-54; and Loe et al., FASEB Journal, 2001,15 4:632, Abs. 501.1). Body weight of the test subjects as well as, blood levels of lipids, hormones and metabolic indicat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com