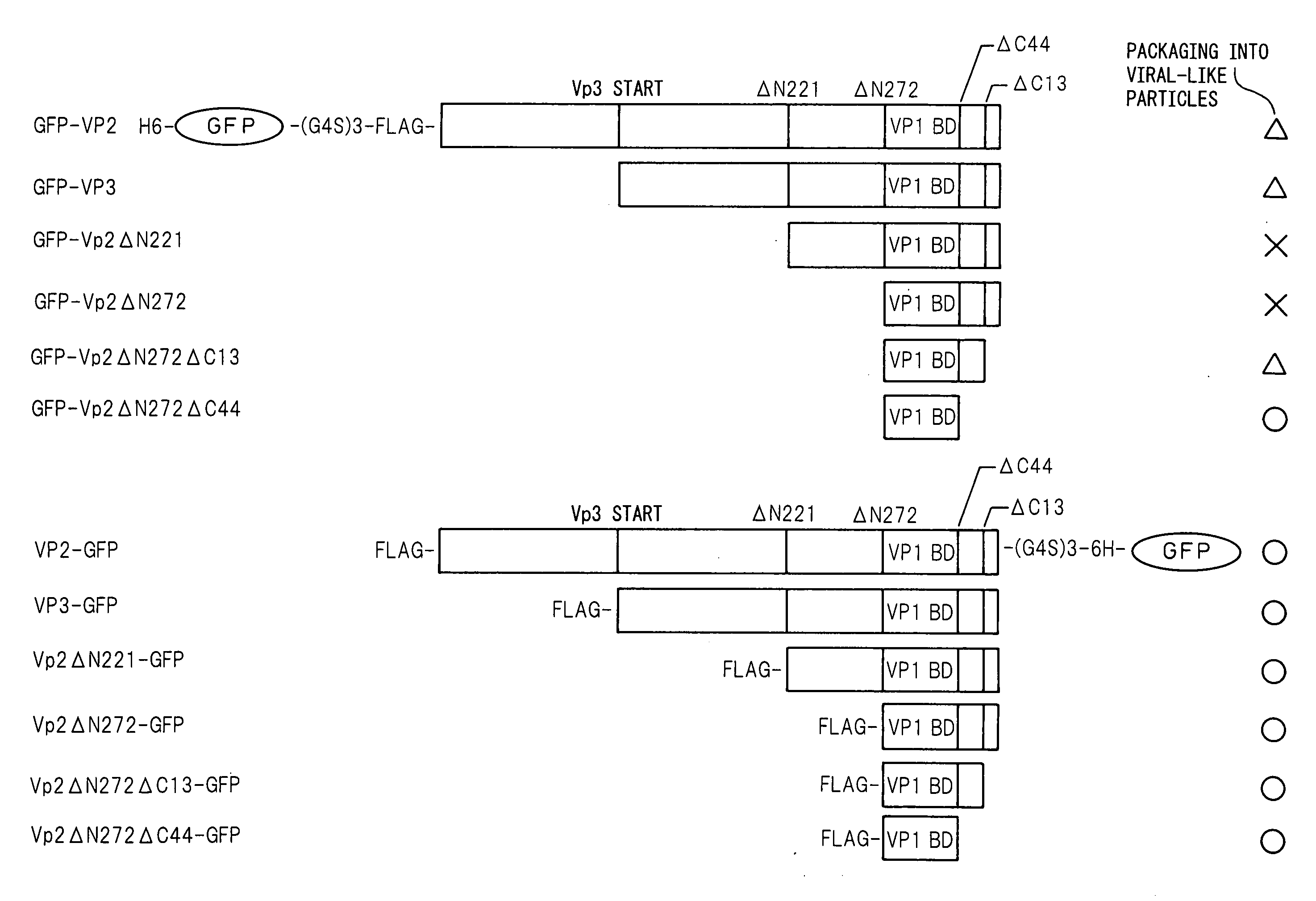
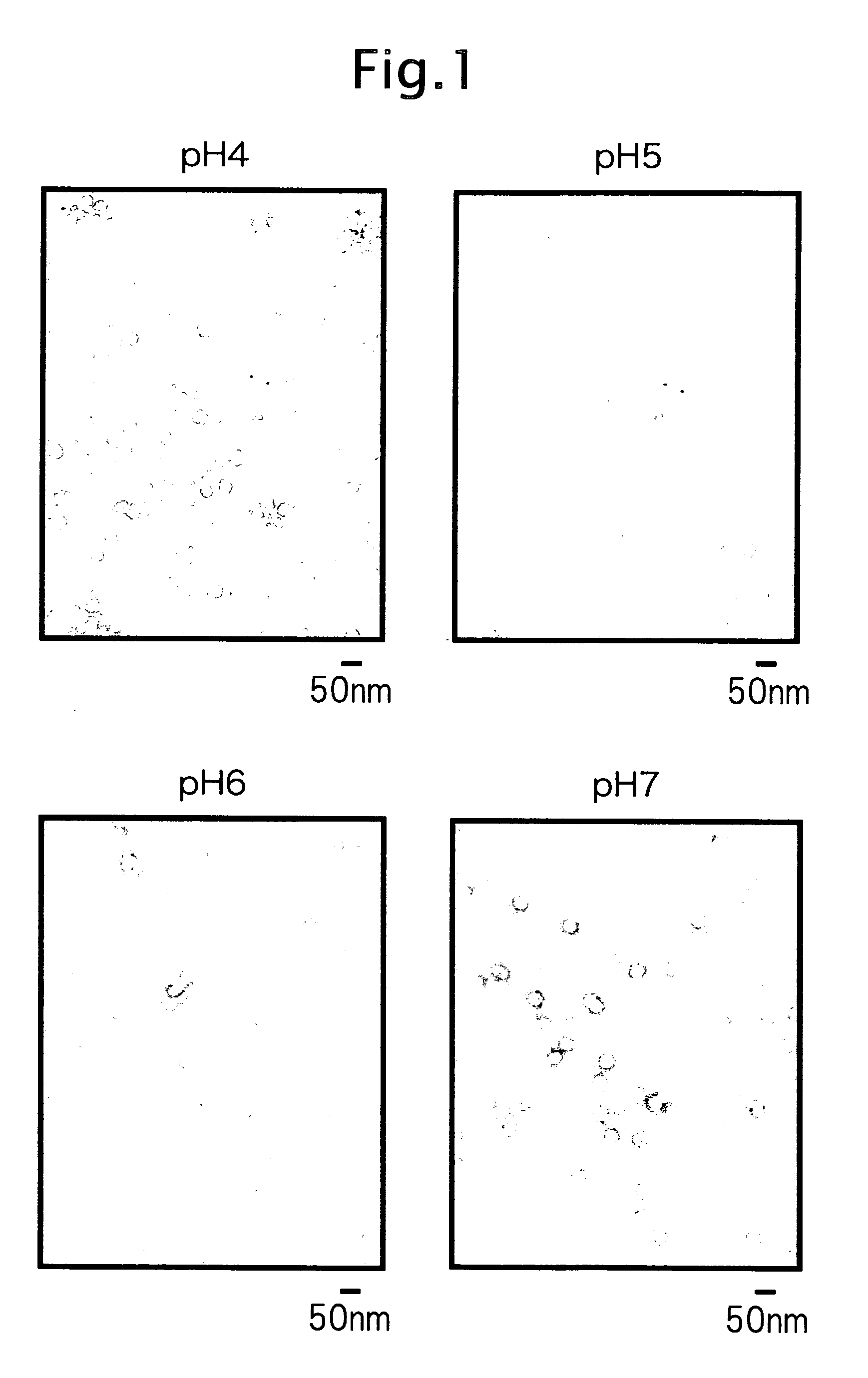
Viral Particle-Like Construct and Method of Forming the Same Under Physiological Conditions
a technology of virus-like particles and constructs, which is applied in the field of virus-like structure, can solve the problems of difficult to efficiently reconstitute virus-like particles of uniform size by such methods, and the conditions are not suitable for taking up bioactive substances into particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Viral Particles
(1) Preparation of Viral Particle (VP1) Pentamers
[0057]After seeding Sf9 cells at 1×107 each into fifty 10 cm-diameter tissue culturing dishes, they were infected with recombinant baculovirus expressing SV40 viral protein (VP1) with a m.o.i. (multiplicity of infection) of 5 to 10.
[0058]At 72 hours after infection, the cells were recovered with medium using a scraper, and rinsed twice with cooled phosphate buffered saline (PBS). To the recovered cells there was added 10 mL of ice-cooled sonication buffer (20 mM Tris-HCl (pH 7.9), 1% (w / v) sodium deoxycholate (DOC), 2 mM PMSF), and then a VP-15S (sonicator) by Taitec was used for 10 minutes of ultrasonic disruption while cooling on ice under conditions with a 50% duty cycle and output at 5. The cell disruptate was centrifuged at 14,000 g, 4° C. for 20 minutes and the supernatant was recovered.
[0059]Cesium chloride solutions with four different densities (50%, 40%, 30%, 20% (w / v)) were gently layered at 1....
example 2
Formation of Virus-Like Particles Incorporating DNA
[0073]Example 1 was repeated. However, in step (3) for in vitro reconstitution of the virus-like particles under physiological conditions, a 3000 bp plasmid was included and the formed virus-like particles comprising DNA incorporated into the virus-like particles were subjected to sucrose density gradient centrifugation, and then fractionation and Southern blotting for detection of DNA. As shown in FIG. 7, the DNA was incorporated into the virus-like particles.
[0074]The prepared SV40-VP1 protein pentamers and SV40-VP2 protein were used for in vitro reconstitution of the virus-like particles under physiological conditions. DNA was added during the procedure. Specifically, at pH 5.0 to 7.0, for example, 2.8 μL of 800 ng / μL VP2 protein was added to 150 μL of 75.7 ng / μL VP1 pentamer protein, and then 21 μL of 5.7 ng / μl 3000 bp circular double-stranded plasmid DNA (pG5vector) was added. The mixture was incubated at 4° C. for 30 minutes a...
example 3
Gene Transfer into Cells Using DNA-Incorporating Virus-Like Particles
[0076]Example 2 was repeated. However, the plasmid used was pEG which can express a fluorescent protein (GFP) in mammalian eukaryotic cells. Formation of virus-like particles containing the pEG plasmid DNA was accomplished by sucrose density gradient centrifugation, and it was confirmed that DNA was contained in the fractions containing the virus-like particles. Specifically, the virus-like particles were reconstituted in a solvent containing 150 mM sodium chloride, 2 mM calcium chloride and 20 mM Tris HCl (pH 7.2) using VP1 and VP2 protein in the presence of the pEG plasmid, and the plasmid DNA-containing virus-like particles were fractionated by sucrose density gradient centrifugation.
[0077]The virus-like particles were detected by Western blotting using anti-VP1 antibody (a-VP1), and pEG was detected by Southern blotting. The results are shown in FIG. 8. The numbers in the image represent the fraction numbers, w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com