Chondroitin sulfate synthesis promoter
a synthesis promoter and chondroitin technology, applied in the direction of transferases, peptide/protein ingredients, drug compositions, etc., can solve the problems of significant impairment of shock absorption performance and friction reduction performance (lubricating action), and achieve the effect of improving cartilage functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
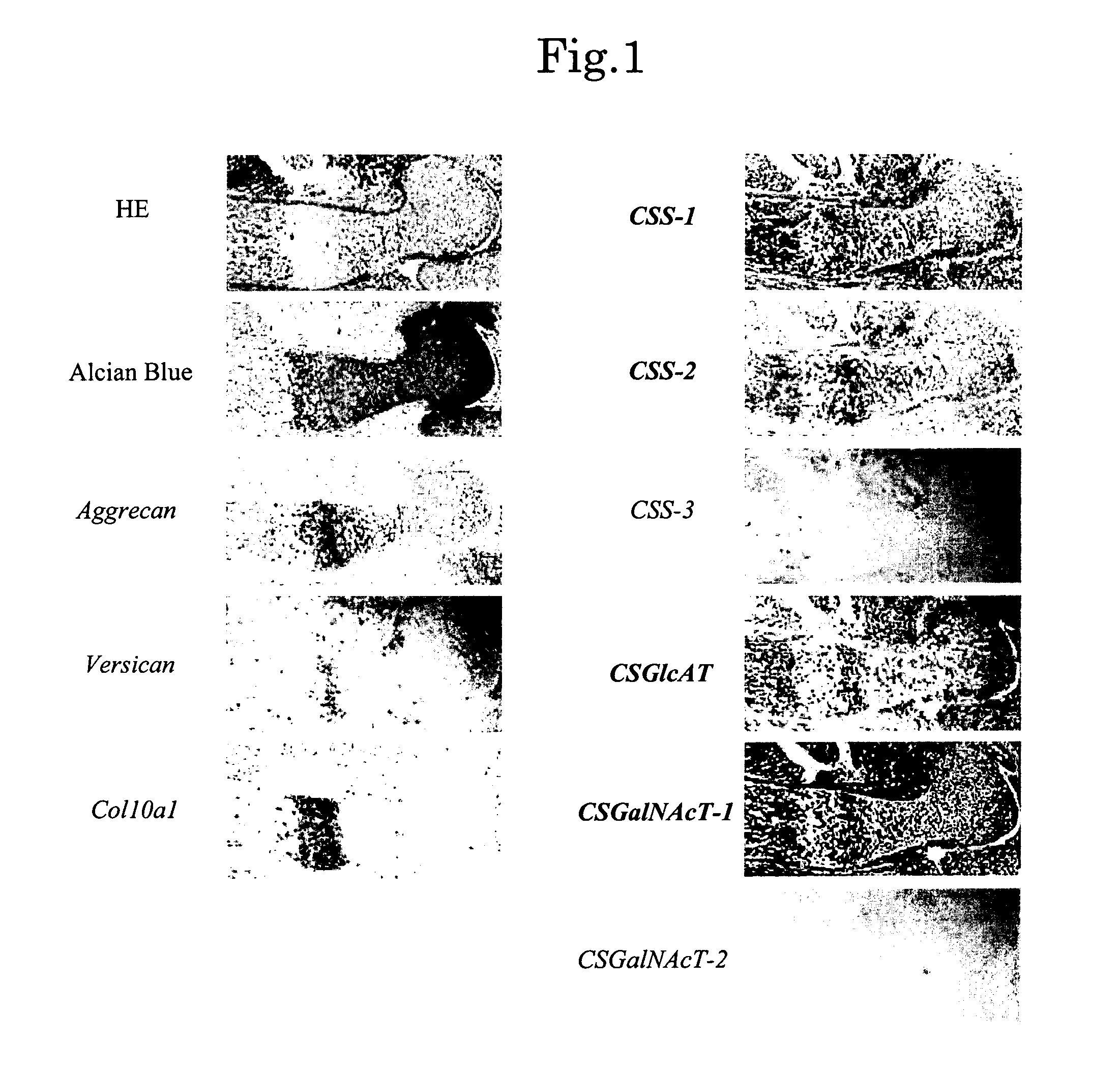
Expression of Chondroitin Sulfate Glycosyltransferase in Cartilage
[0112]Humeri were obtained from mouse E16.5 embryos, and in situ hybridization was performed in order to confirm expression of chondroitin sulfate glycosyltransferase in cartilage.
[0113]Digoxigenin (DIG)-11-UTP-labeled single-strand antisense RNA probes corresponding to mouse CSS-1, CSS-2, CSS-3, CSGlcAT, CSGalNAcT-1, CSGalNAcT-2, aggrecan, versican, and type-10 collagen α1 chain (Col10a1) were prepared by use of a DIG RNA labeling kit (product of Boehringer Mannheim) according to the manufacturer's instructions. Each humerus sample was collected from a mouse E16.5 embryo, and a paraffin section thereof was prepared. After removal of paraffin, the section was fixed with 4% paraformaldehyde for 10 minutes and treated with 20-ng / mL protein kinase K (product of Roche) at 37° C. for 7 minutes. Another fixation was performed with 4% paraformaldehyde for 10 minutes, followed by treating with 0.2-mol / L hydrochloric acid for ...
example 2
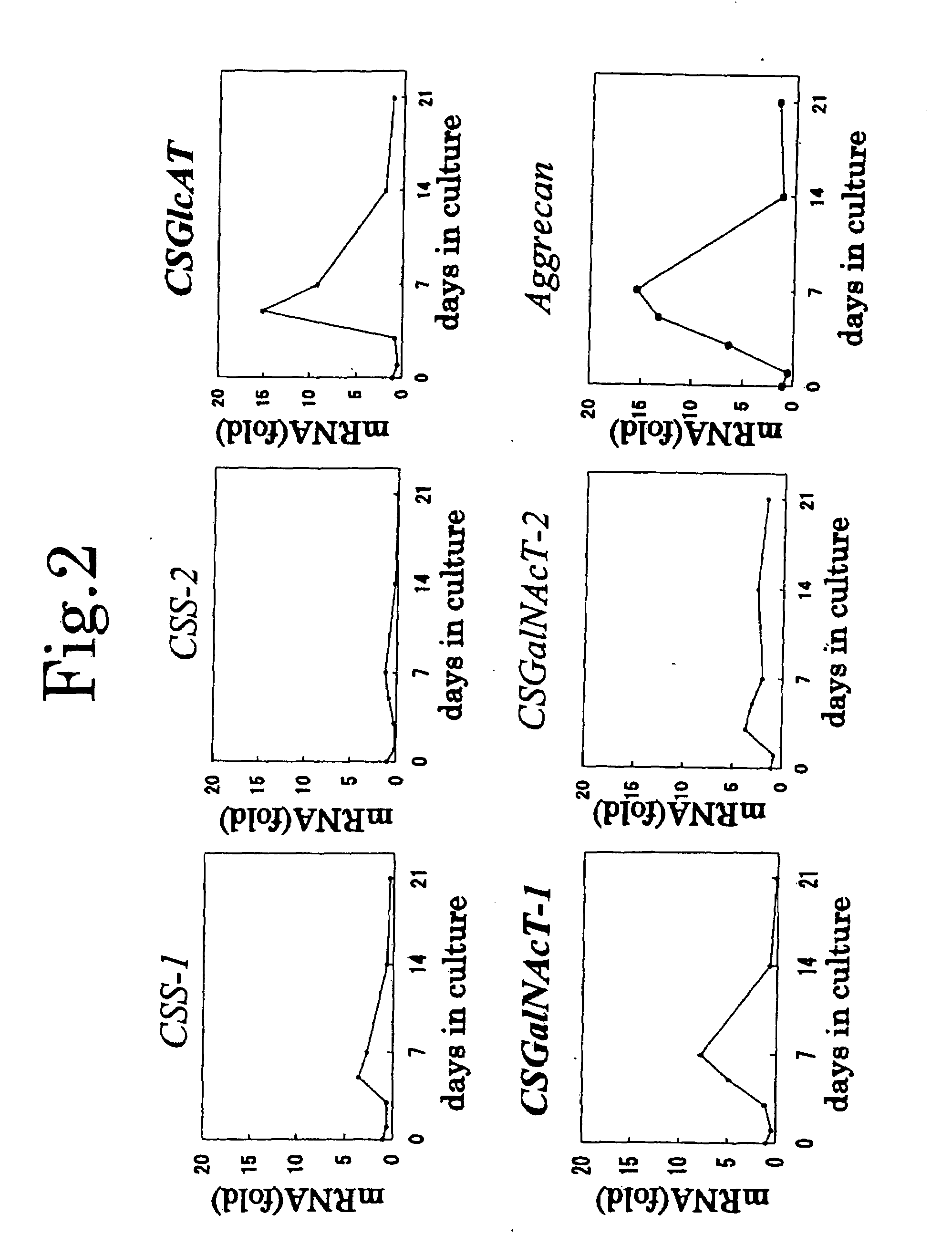
Expression Patterns of Chondroitin Sulfate Glycosyltransferases During Chondrocyte Differentiation
[0116]Chondrogenic ATDC5 cells were cultured in Dulbecco's modified Eagle medium (DMEM) / F-12 medium containing 5% fetal bovine serum (FBS), penicillin and streptomycin. In order to induce differentiation of chondrocytes, the cells at confluency were treated with 10-μg / mL bovine insulin, 10-μg / mL human transferrin, and 3×10−8-mol / L sodium selenite.
[0117]On days 1, 3, 5, 7, 14, and 21 after induction of differentiation (day 0: at about 80% confluency (growing phase) before induction of differentiation), the expression amount of mRNA (a gene transcription product) corresponding to mouse CSS-1, CSS-2, CSS-3, CSGlcAT, CSGalNAcT-1, CSGalNAcT-2, or aggrecan was determined through real time RT-PCR.
[0118]mRNA was extracted by use of Micro-FastTrack (product of Invitrogen), and cDNA was reverse transcripted therefrom by use of SuperScript First-Strand (product of Invitrogen). The primers and fluo...
example 3
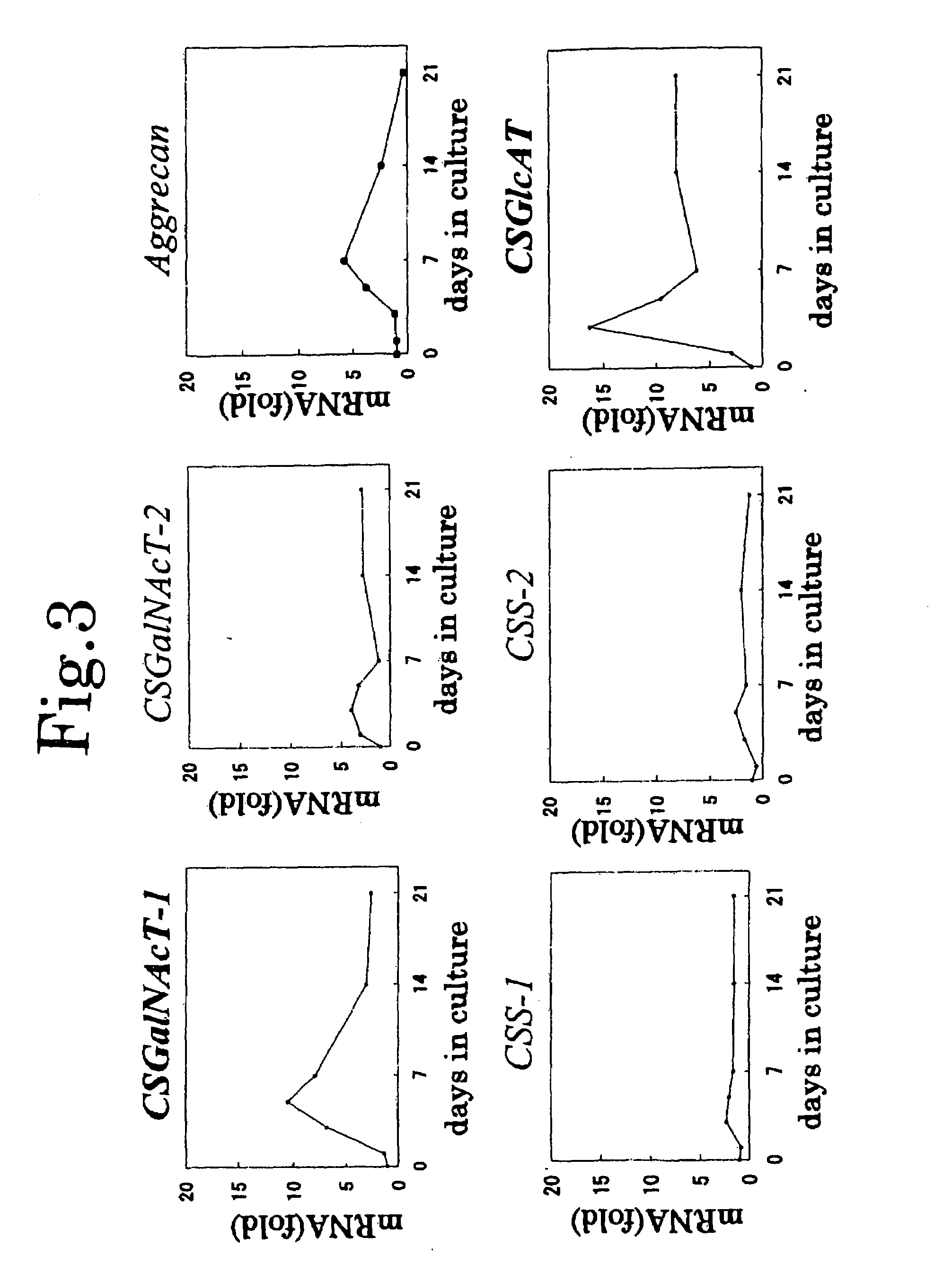
Expression Patterns of Chondroitin Sulfate Glycosyltransferases During Chondrocyte Differentiation
[0121]Chondrogenic N1511 cells were cultured in a minimum essential medium α (α-MEM medium) supplemented with 10% FBS, penicillin, and streptomycin at 37° C. under 5% CO2. The cells at confluency were treated with 1×10−6-mol / L dexamethasone solution and 1×10−7-mol / L rat parathyroid hormone (PTH) solution, to thereby induce differentiation. In a manner similar to that of Example 2, on days 1, 3, 5, 7, 14, and 21 after induction of differentiation (day 0: at about 80% confluency (growing phase) before induction of differentiation), the level of mRNA (a gene transcription product)corresponding to CSS-1, CSS-2, CSS-3, CSGlcAT, CSGalNAcT-1, CSGalNAcT-2, or aggrecan was determined. In a manner similarly to that of Example 2, the aforementioned mRNA levels were standardized by an mRNA level of GAPDH. The thus-standardized mRNA expression levels were plotted as fold numbers with respect to thos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com