Proton acceptance type sensor, hydrogen gas sensor and acid sensor
a sensor and proton acceptance technology, applied in the field of sensors, can solve the problems of limiting the level of miniaturization reducing the amount of hydrogen gas, so as to achieve the effect of favorable protons sensory selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0076] 1. Electrical Resistance Mode
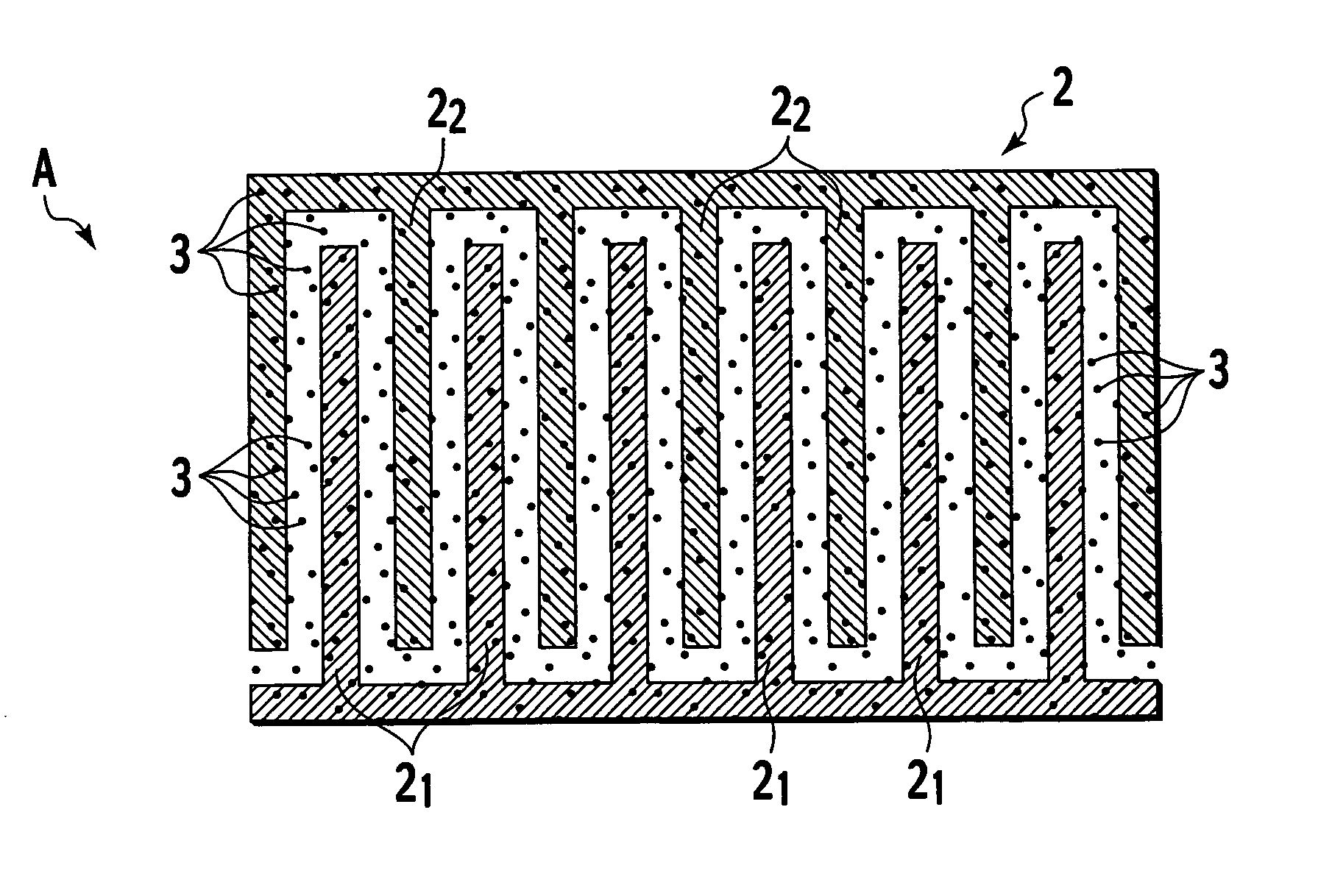
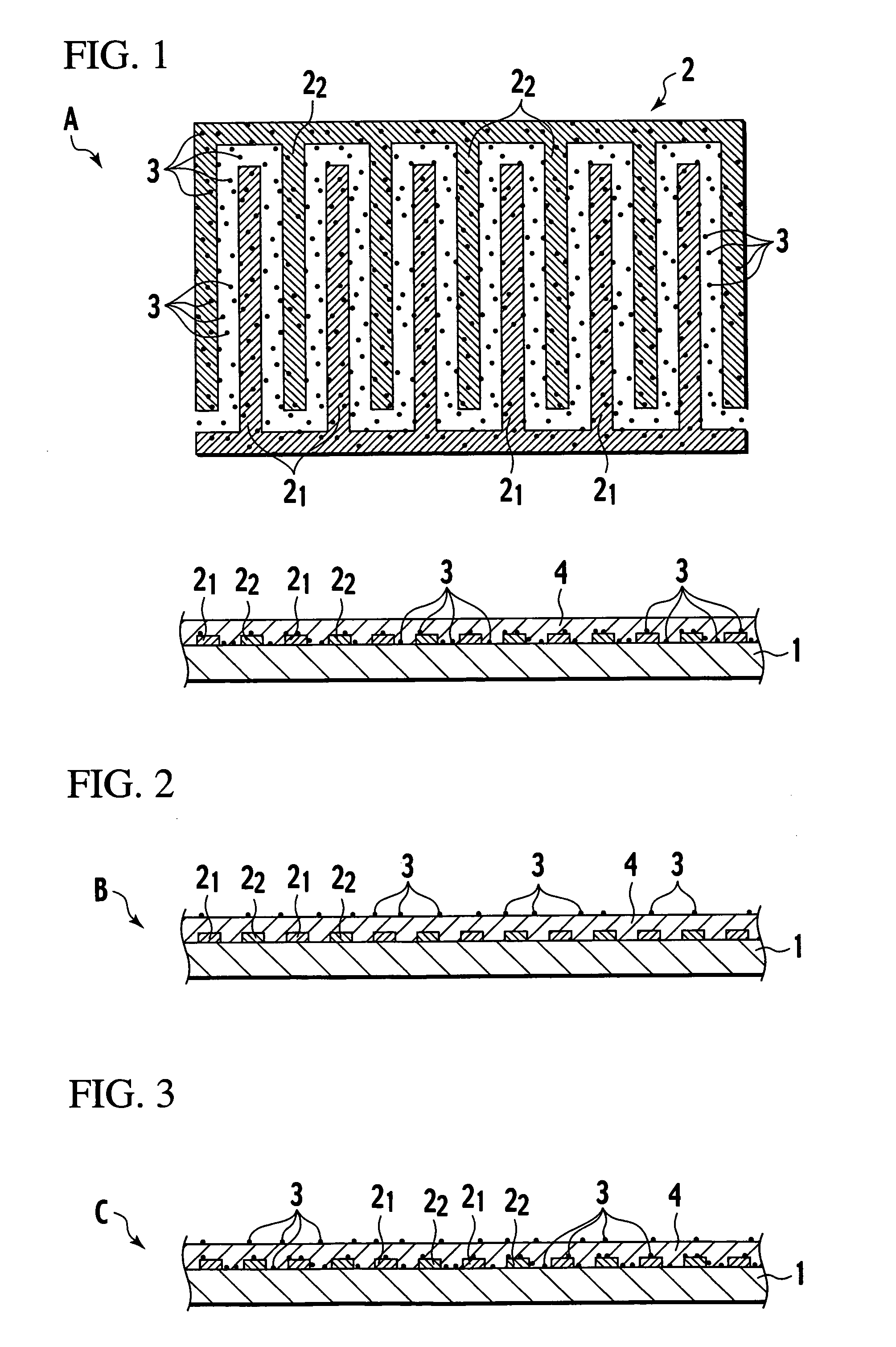
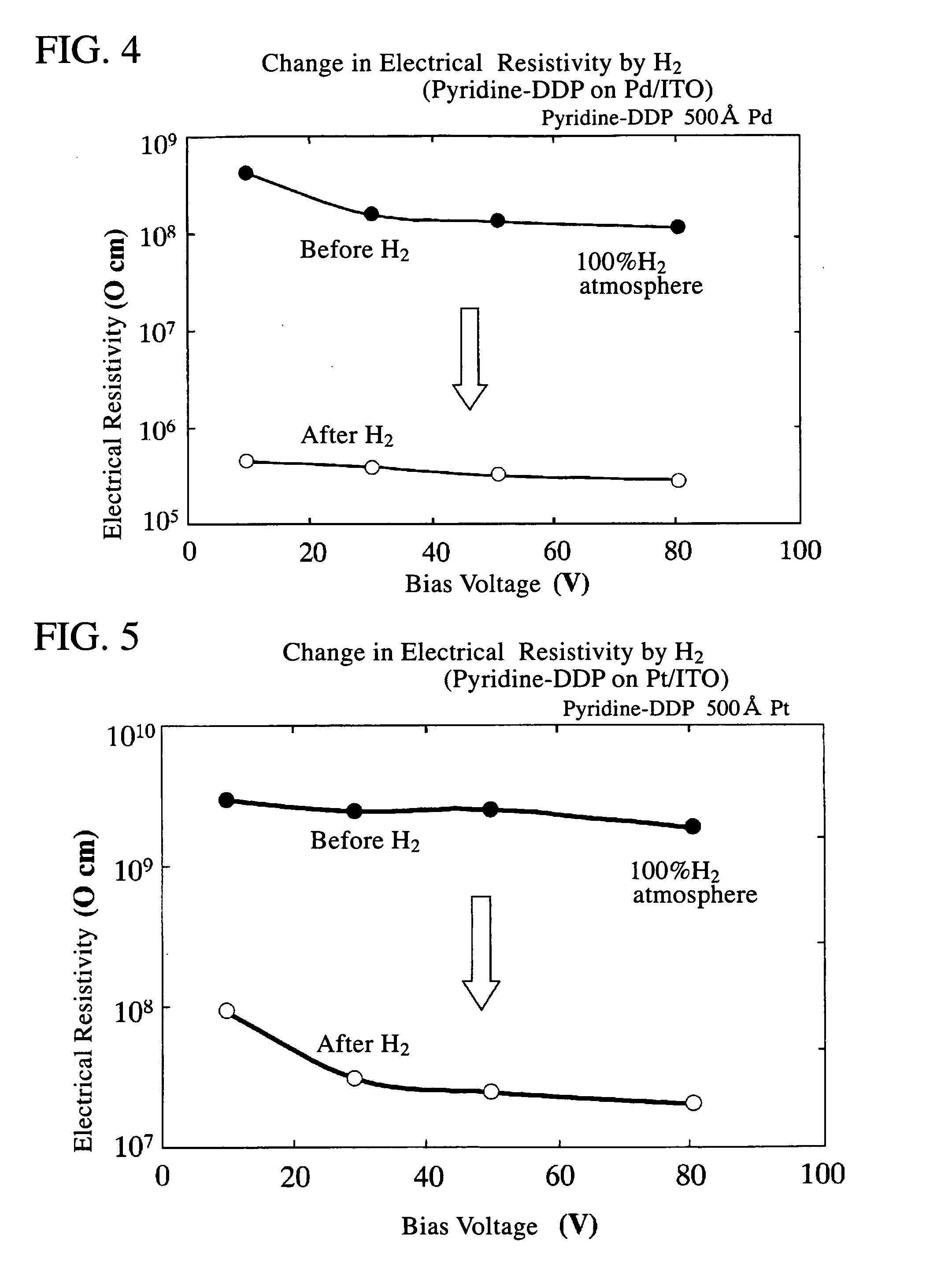
[0077] When an element with the structure shown in FIG. 1 is placed in an atmosphere containing hydrogen gas, nitric acid gas, hydrogen chloride gas, hydrogen fluoride gas, or ammonia gas or the like, the electrical resistivity falls rapidly. Because the electrical resistivity of pyridine-DPP is normally extremely high, and close to that of an insulator, a voltage is applied across the electrodes, and the minute current that flows is detected. In other words, by detecting and amplifying any changes in this minute current, the element can be used as a sensor. In an element of the present invention, the change in electrical resistivity is within a range from 1 to 4 or more orders of magnitude, and is consequently extremely easy to detect. The detection system, namely the input system, is of high impedance, and the circuit design preferably takes this factor into account. For example, an arrangement in which a buffer that uses a high input impedance...
example 2
[0079] 2. Photoconduction Mode
[0080] The structure of the element is the same as that for the electrical resistance mode, with the exceptions that the substrate was a glass plate, and ITO was used as the electrodes. When this element is irradiated with visible light, the electrical resistance decreases dramatically (a photoconduction phenomenon), enabling hydrogen gas to be detected.
example 3
[0081] 3. Absorption Band Mode
[0082] In this optical absorption band mode, electrodes are unnecessary, and the remaining structures are the same as those for the electrical resistance mode and photoconduction mode. When proton addition occurs within the pyridine-DPP film, the 540 nm absorption band shifts to 580 nm, and this mode detects this change in the optical absorption band using a semiconductor detector or a photomultiplier, enabling use as a hydrogen gas sensor.
[0083] Both the electrical resistance mode and the photoconduction mode basically use a change in the electrical resistance as the sensor. The mode of operation can use a detection method that uses either a direct current or an alternating current.
Industrial Applicability
[0084] The present invention provides a low-cost proton acceptance type gas sensor, and in particular a hydrogen gas sensor, which exhibits favorable sensory selectivity for protons, can use a variety of different detection devices, detects change...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com