Remedy for spinal injury containing interleukin-6 antagonist
a technology of interleukin-6 and spinal cord injury, which is applied in the direction of antibody medical ingredients, drug compositions, peptide/protein ingredients, etc., can solve the problems of side effects, no effective treatment methods are currently available, and no results that indicate recovery from paralysis, etc., and achieve the effect of restoring motion coordination and spinal cord recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
Animals:
[0137] Adult (18-22 g) female c57BL / 6J mice were used in all experiment groups.
Spinal Cord Injury:
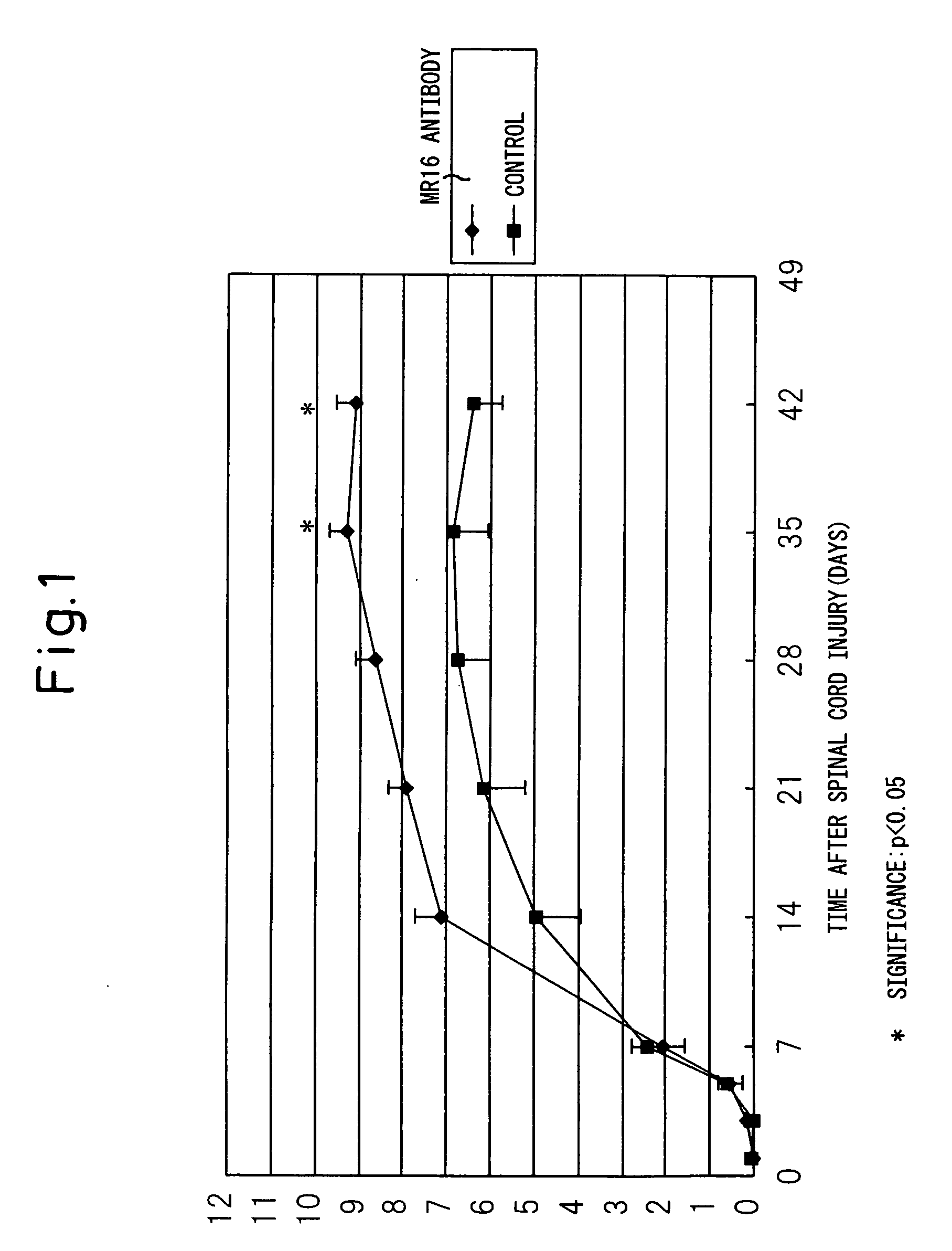
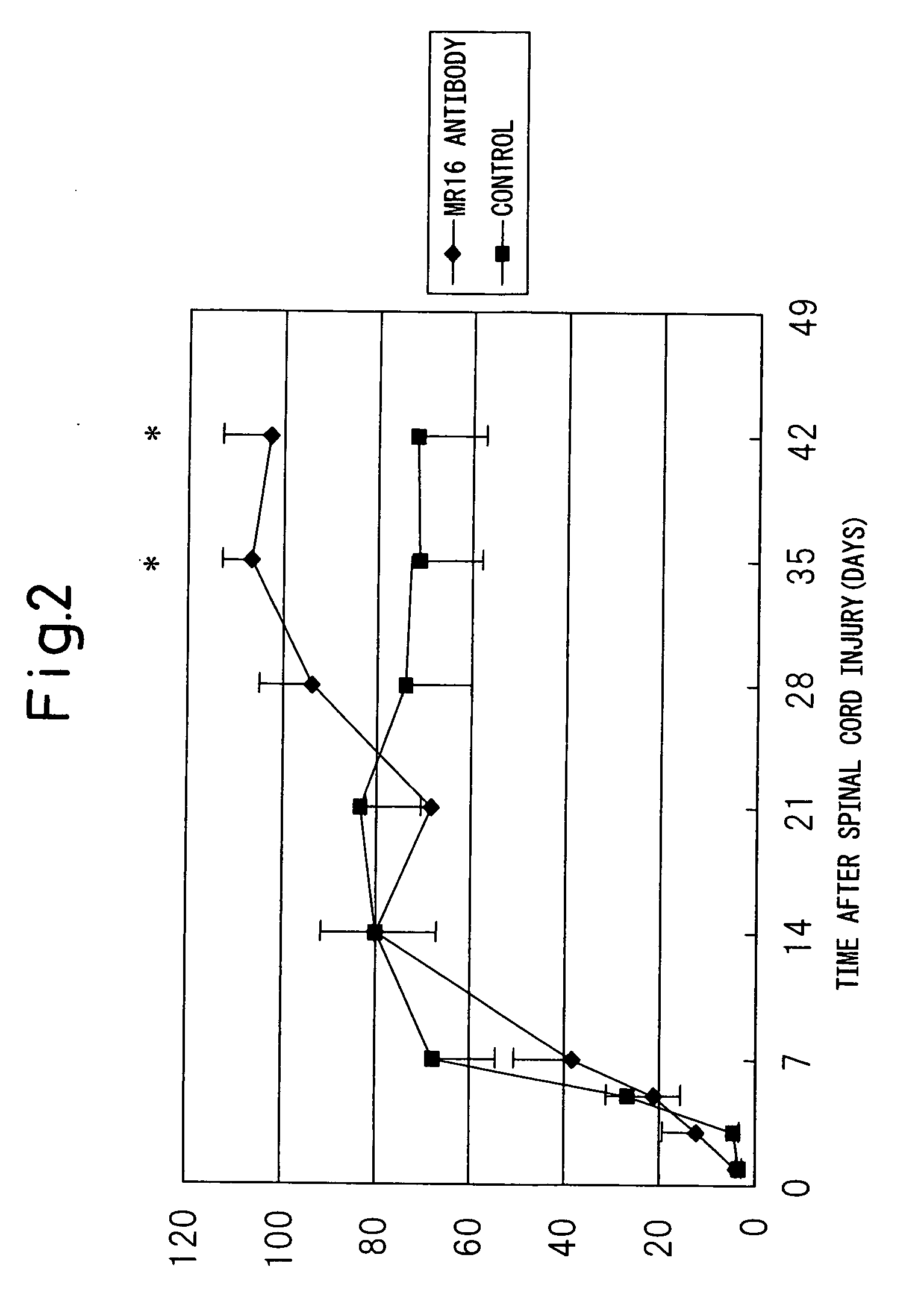
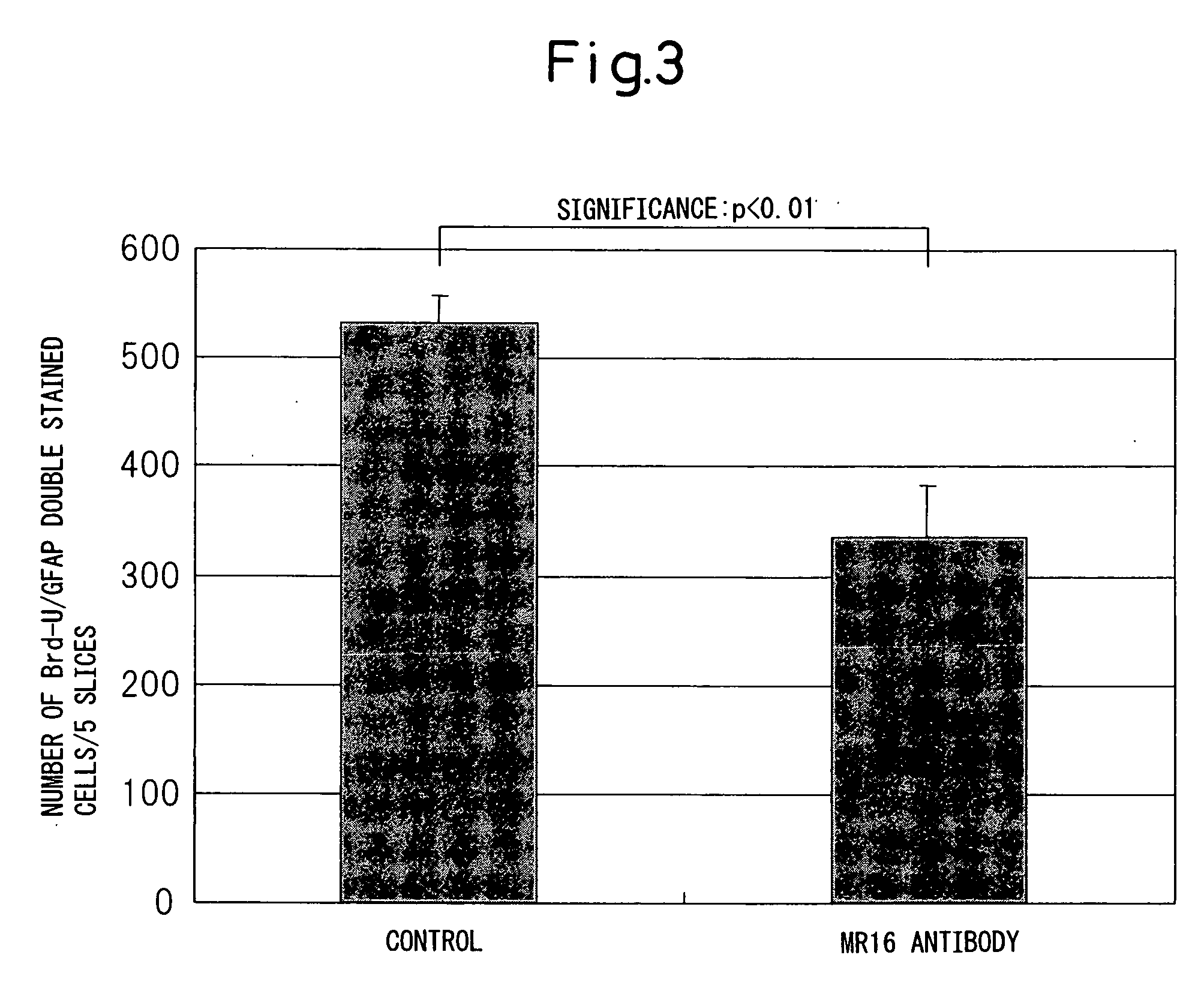
[0138] Female mice were anesthetized by the intraperitoneal injection of ketamine (100 mg / kg) and xylazine (10 mg / kg). The back was shaved, a 20 mm midline skin incision was made, and then the spine was exposed. After the thoracic region of the spine was exposed by separation to the sides of the back muscle, the spinous process of the T7-T13 vertebra was exposed. Laminectomy was made at the ninth thoracic spine level to expose the spinal cord taking care not to injure the dura mater. The spine was stabilized with forceps and clamps on the T7 and T11 spinous processes and the ligament. After the animal body was floated by lowering the stage, spinal cord injury (SCI) was made by the NYU impactor. A 3 g weight (the apex with a diameter of 1.2 mm) was dropped from a height of 25 mm to the T9 level spinal cord. Muscles and the incised part were closed in l...
reference example 1
Preparation of Human Soluble IL-6 Receptor
[0153] Soluble IL-6 receptor was prepared by the PCR method using a plasmid pBSF2R.236 containing cDNA that encodes IL-6 receptor obtained according to the method of Yamasaki et al., (Yamasaki, K. et al., Science (1988) 241, 825-828). Plasmid pBSF2R.236 was digested with a restriction enzyme Sph I to obtain the cDNA of IL-6 receptor, which was then inserted into mp18 (manufactured by Amersham). Using a synthetic oligoprimer designed to introduce a stop codon into the cDNA of IL-6 receptor, a mutation was introduced into the cDNA of IL-6 receptor by the PCR method using the in vitro Mutagenesis System (manufactured by Amersham). The procedure resulted in the introduction of a stop codon to the amino acid at position 345, and gave cDNA encoding soluble IL-6 receptor.
[0154] In order to express the cDNA of soluble IL-6 receptor in CHO cells, it was ligated to a plasmid pSV (manufactured by Pharmacia) to obtain a plasmid pSVL344. The cDNA of so...
reference example 2
Preparation of Anti-Human IL-6
[0157] antibody
[0158] Ten μg of the recombinant IL-6 (Hirano et al., Immunol. Lett., (1988) 17, 41) was used for immunizing BALB / c mice together with Freund's complete adjuvant, and this was repeated every week until anti-IL-6 antibody could be detected in the serum. Immune cells were extracted from local lymph nodes and were then fused with a myeloma cell line P3U1 using polyethylene glycol 1500. Hybridomas were selected according to the method of Oi et al. (Selective Methods in Cellular Immunology, W.H. Freeman and Co., San Francisco, 351, 1980) that employs the HAT medium, and the hybridoma that produces anti-human IL-6 antibody was established.
[0159] The hybridoma that produces anti-human IL-6 antibody was subjected to the IL-6 binding assay as follows. Thus, a 96-well microtiter plate made of flexible polyvinyl (manufactured by Dynatech Laboratories, Inc., Alexandria, Va.) was coated with 100 μl of goat anti-mouse Ig (10 μl / ml, manufactured by C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Therapeutic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com