Catalytic sulfur removal from a hydrocarbon stream
a hydrocarbon stream and catalytic technology, applied in the direction of separation processes, dispersed particle separation, chemistry apparatus and processes, etc., can solve the problems of reducing the production of aromatic hydrocarbons, limiting the formation of coke on the catalyst, and affecting the quality of gasoline, so as to reduce the size of the reactor, short residence time, and high conversion of sulfur compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
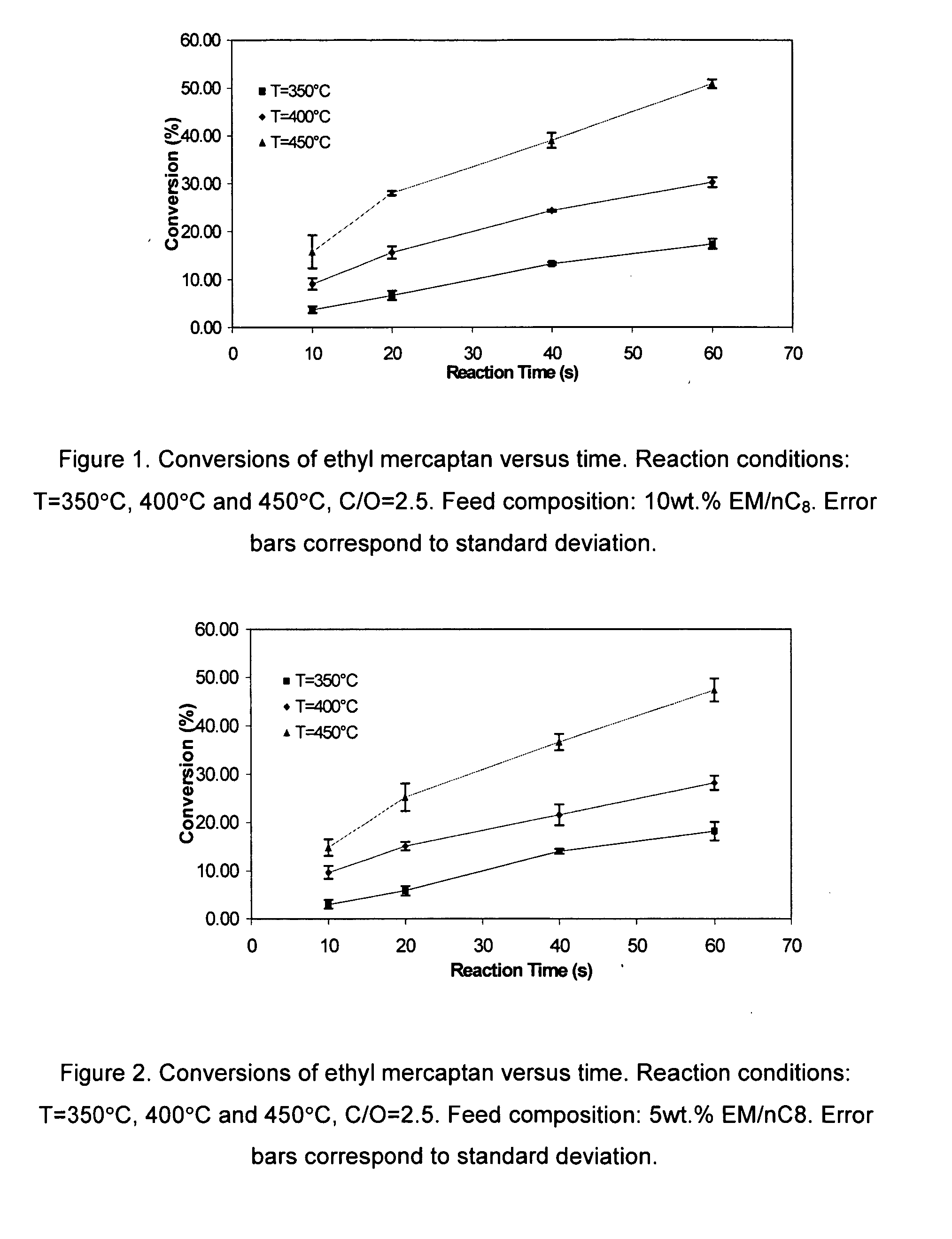
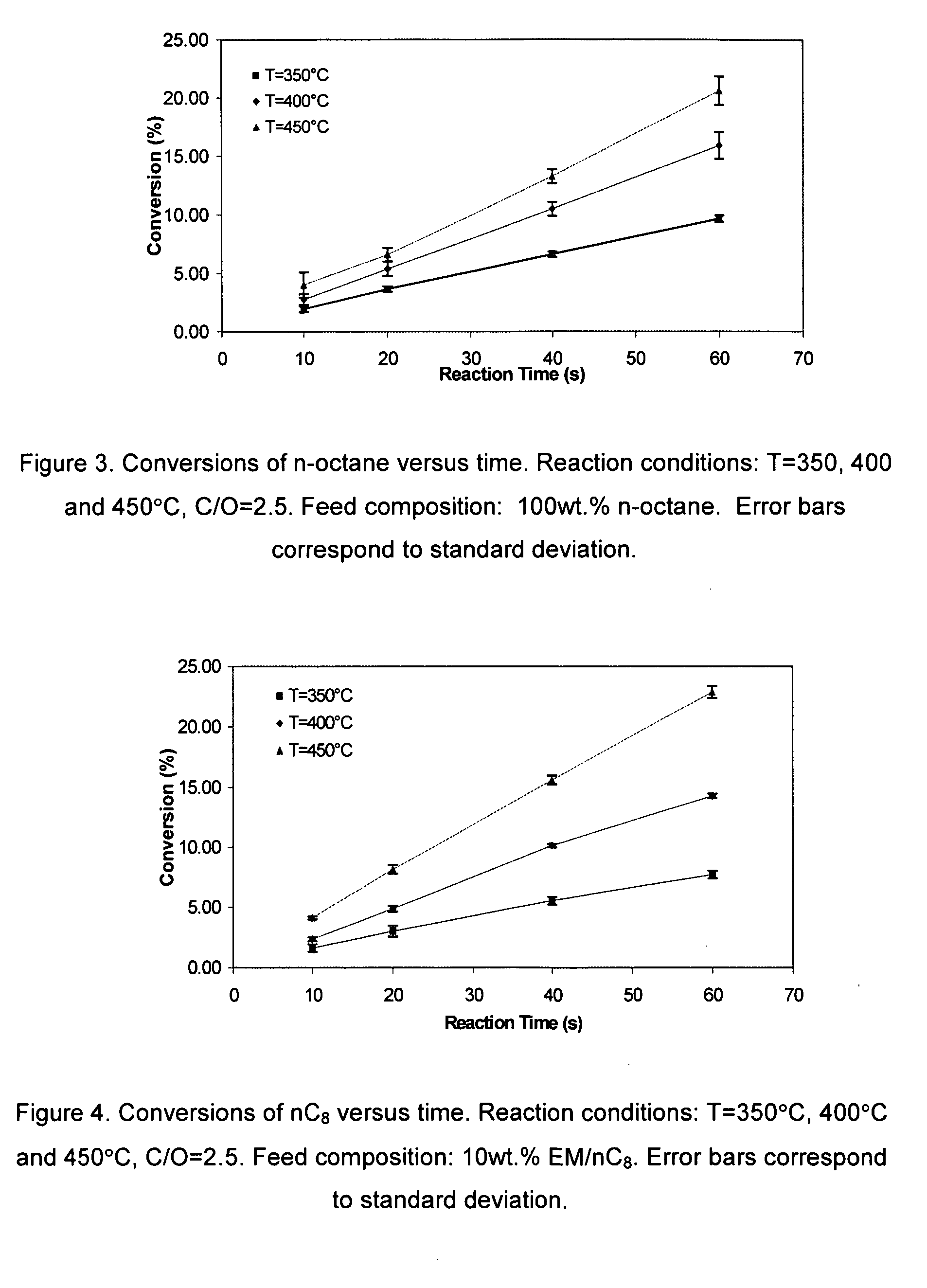
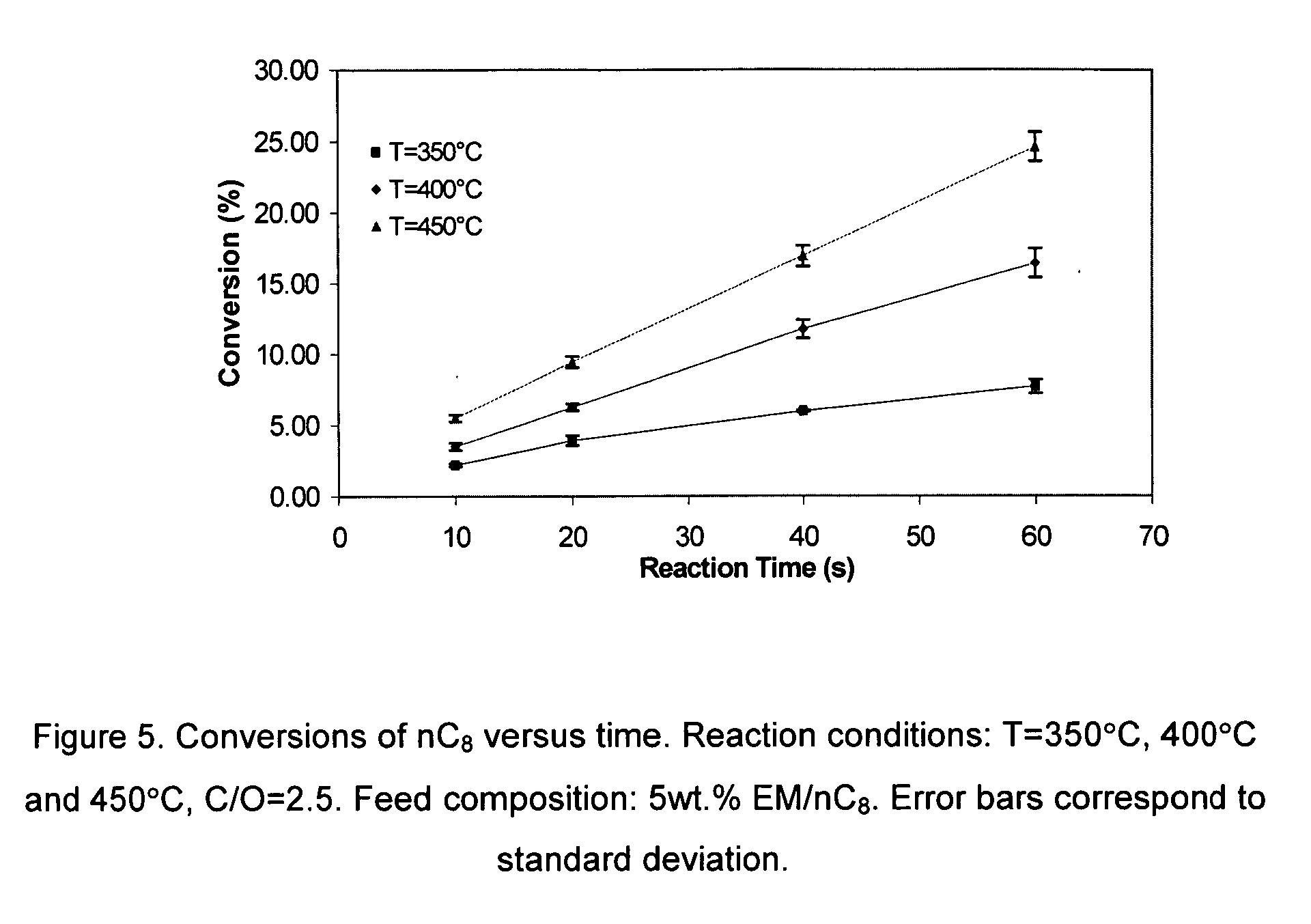
[0039] Ethyl mercaptan reacts over H-ZSM5 as follows: [0040] 1. Intra-molecular dehydrosulfidation. Ethyl mercaptan reacts via intra-molecular dehydrosulfidation to give ethylene and H2S,
CH3−CH2SH⇄CH2=CH2+H2S (1)[0041] 2. Inter-molecular dehydrosulfidation between two mercaptan reacting molecules. This reaction leads to the removal of H2S molecule from two mercaptan molecules yielding diethyl sulfide (DiE-S) and H2S:
CH3−CH2SH+CH2SH−CH3⇄CH3−CH2−S−CH2−CH3+H2S (2)[0042] Moreover, following, this first step, a second step involves further intra-molecular dehydrosulfidation of diethyl sulfide (DiE-S) yielding an olefin (butene) and H2S:
CH3−CH2−S−CH2−CH3⇄CH3−CH2=CH2−CH3+H2S (3)
[0043] Equilibrium constants and equilibrium compositions of the proposed set of three simultaneous reactions for the dehydrosulfidation of ethyl mercaptan (EM) can be considered at set operation conditions: temperature, pressure, reactant concentration. The theoretically calculated chemical equilibrium consta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| residence time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com