Antimicrobial polymeric surfaces
a technology of polymeric surfaces and antimicrobials, applied in the field of antimicrobial polymeric surfaces, can solve the problems of inability to be effective against airborne bacteria, prone to impotence, frequent infection, etc., and achieve the effect of preventing the formation of biofilms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Method A
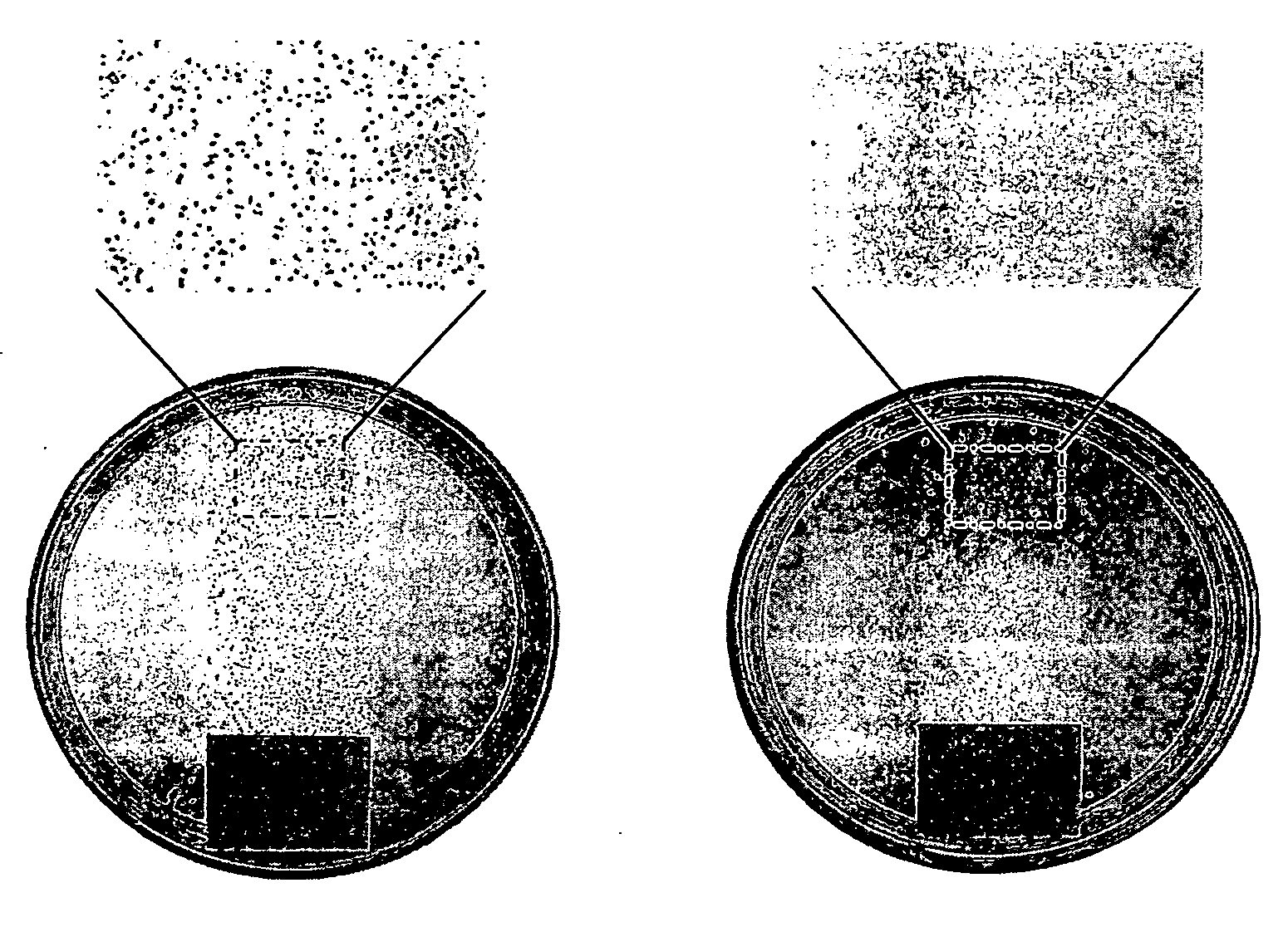
[0173] A NH2-glass slide (aminopropyltrimethoxysilane-coated microscopic slides) was placed in 90 mL of dry dichloromethane containing 1 mL of triethylamine. After cooling to 4° C., 10 mL of acryloyl chloride was added, and the reaction mixture was stirred in a cold room overnight and then at room temperature for 2 h. The acylated NH2-glass slides were rinsed with a methanol / triethylamine mixture (1:1, v / v) and methanol. As judged from the determination of the NH2 groups on the glass slide surface before (6.6±0.1×10−10 mol / cm2) and after (3.3±0.2×10−10 mol / cm2) the acryloylation using the picric acid titration (17), some half of the surface-bound amino groups reacted with acryloyl chloride. The glass-bonded acryloyl moieties were then copolymerized with 4-vinylpyridine. Perchloric acid (90 mL of a 20% solution in water) was degassed, and 30 mg of Ce(SO4)2 was added under argon. After 1 h of stirring, an acryloylated glass slide was placed in this solution, 15 mL of freshly...
example 2
Method B
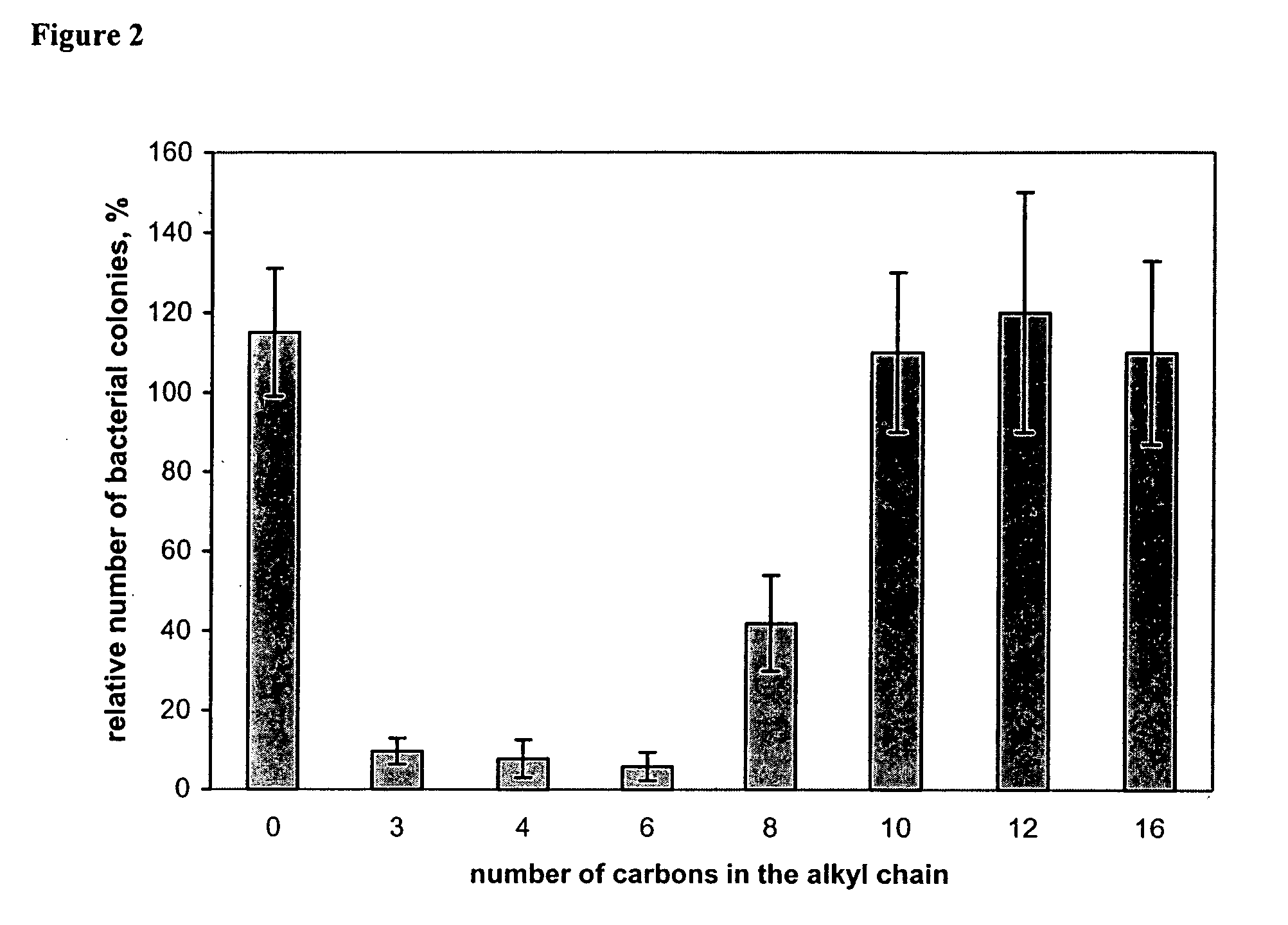
[0174] A NH2-glass slide was immersed in a mixture containing 9 mL of 1,4-dibromobutane, 90 mL of dry nitromethane, and 0.1 mL of triethylamine. After stirring at 60° C. for 2 h, the slide was removed, thoroughly rinsed with nitromethane, air dried, and placed in a solution of 9 g of PVP (molecular weight of 60,000 or 160,000 g / mol) in 90 mL of nitromethane / hexyl bromide (10:1, v / v). After stirring the reaction mixture at 75° C. for 24 h, the slide was rinsed with acetone, thoroughly washed with methanol (to remove the non-attached polymer), and air dried. According to the literature (14), more than 96% of the pyridine rings of PVP should be N-alkylated under these conditions.
Surface Analysis
example 3
[0175] A chemically modified glass slide was immersed in a 1% solution of fluorescein (Na salt) in distilled water for 5 min. Under these conditions, the dye binds to quaternary amino groups (20), but not to tertiary or primary ones (we found that PVP-modified or NH2-glass slides do not adsorb fluorescein). After rinsing with distilled water, a stained slide was placed in 25 mL of the 0.1% detergent cetyltrimethyl-ammonium chloride in distilled water and shaken for 10 min to desorb the dye. The absorbance of the of the resultant aqueous solution was measured at 501 nm (after adding 10% of a 100 mM aqueous phosphate buffer, pH 8.0). The independently determined extinction coefficient of fluorescein in this solution was found to be 77 mM−1 cm−1.
[0176] From staining different hexyl-PVP-films (160,000 g / mol, degree of alkylation >95%) with a known polymer content, the stoichiometry of fluorescein binding was found to be approximately 1 dye molecule per 7 hexyl-PVP monomer units. The fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| contact angle | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com