Process for producing a pharmaceutical solid preparation containing a poorly soluble drug
a technology of solid preparation and drug, which is applied in the field of pharmaceutical solid preparation containing a poorly soluble drug, can solve the problems of organic solvent residue, environmental pollution by organic solvents, and safety in operation, and achieve excellent improvement of drug dissolution, improved dissolution, and improved dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
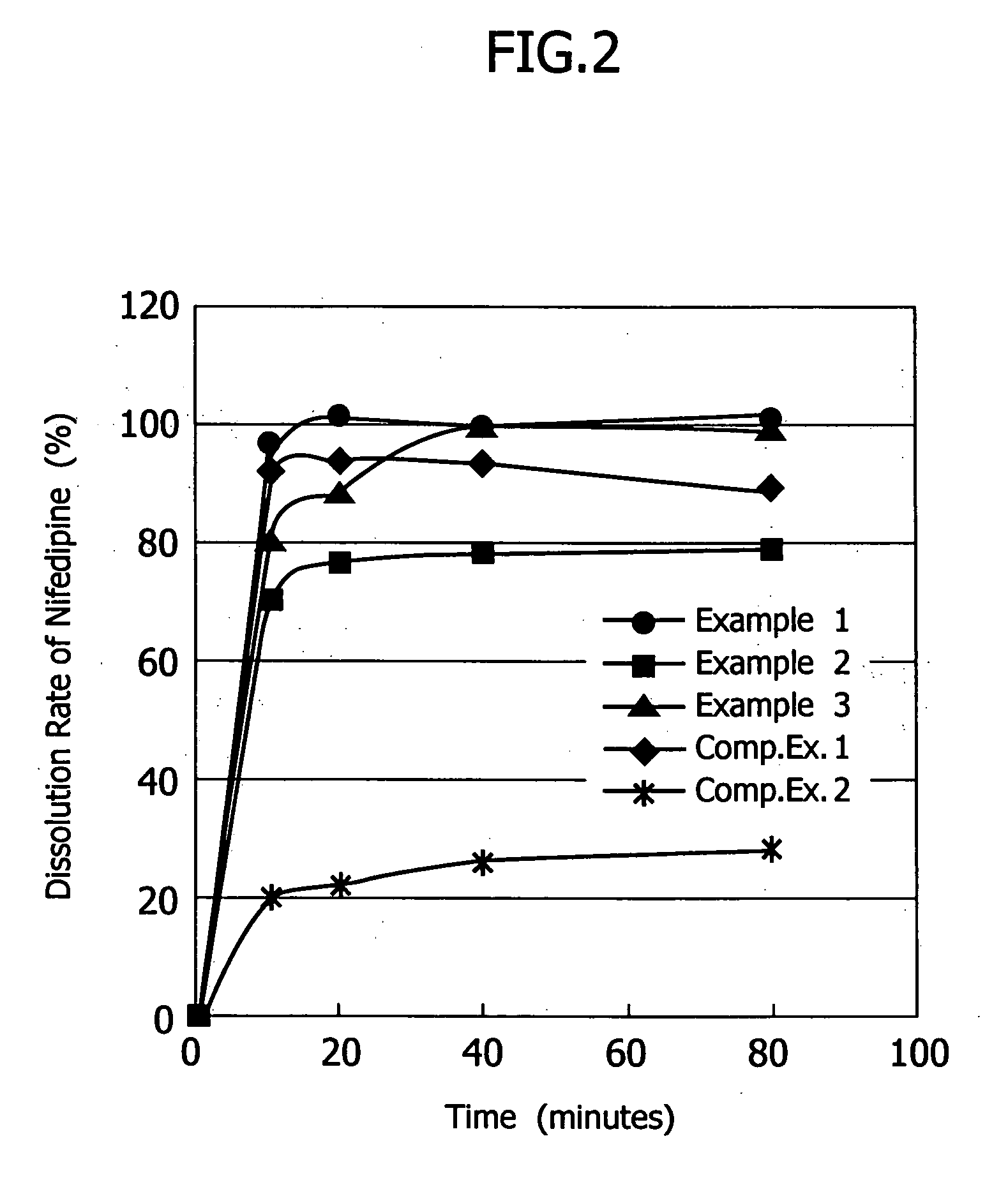
example 1
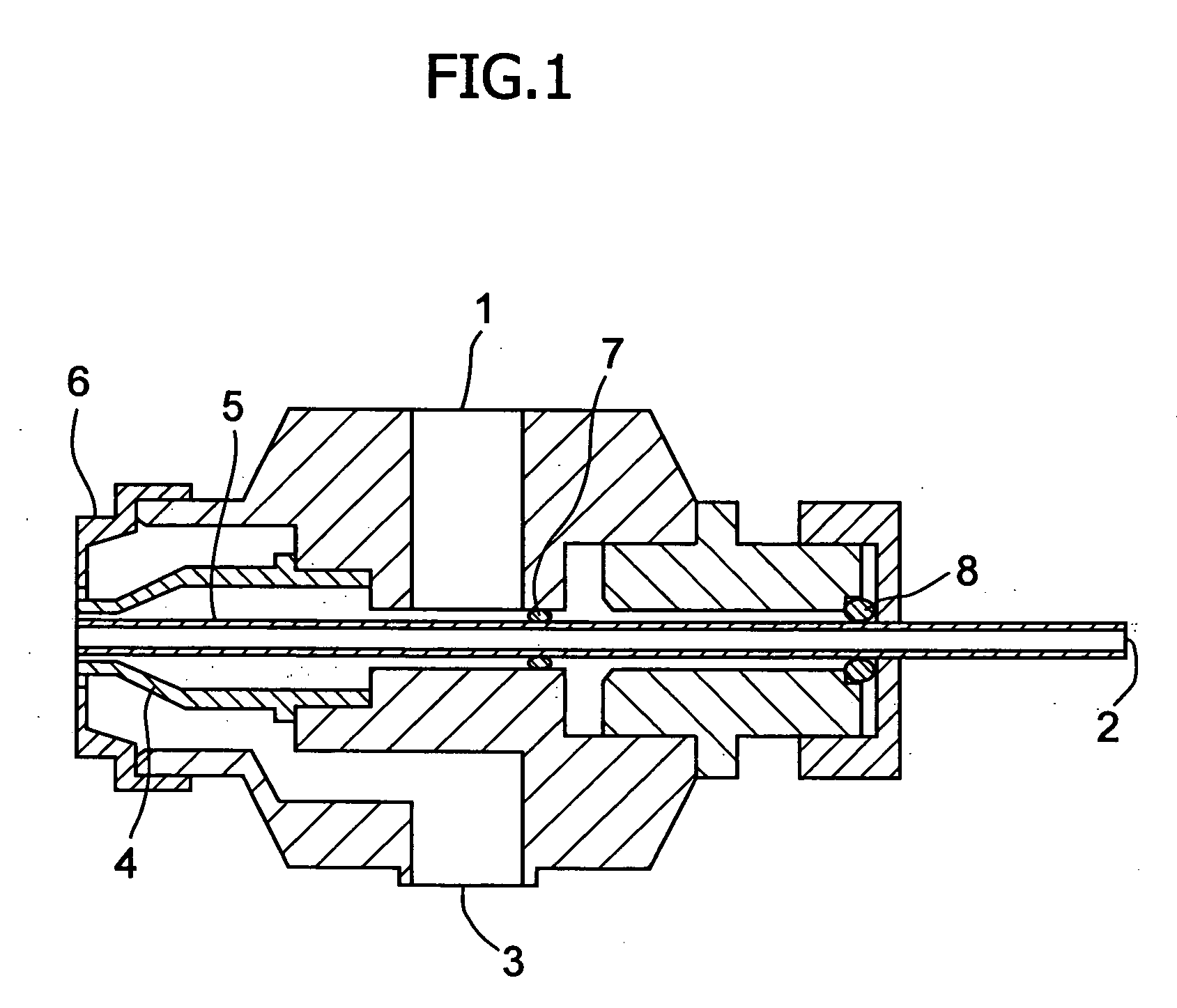
[0032] The poorly soluble drug, nifedipine (10 g)(supplied by Daito Co., Ltd.) was dissolved in a mixture solution of the plasticizer, polyethylene glycol 400 (96 g) and triethyl citrate (4 g) to make Spray A solution. As the water-soluble polymer solution, a dispersion made of 6 g of talc, 0.2 g of sodium lauryl sulfate and 107.1 g of purified water for 20 g of hydroxypropylmethylcellulose acetate succinate (HPMCAS)(AS-MF: supplied by Shin-Etsu Chemical Co. Ltd.) was prepared to make Spray B solution. Lactose (200 g) (lactose 200M: supplied by DMV Co., Ltd.) was fluidized in a fluidized-bed granulation machine (equipment name: Multiplex: supplied by Freund Industrial Co., Ltd.: Multiplex MP-01), and both Spray A and B solutions were sprayed to granulate in a manner of side spraying using three fluid nozzles (spray gun) shown in FIG. 1. Spray A solution, Spray B solution and compressed air were supplied to three fluid nozzles by a tube pump from the outside. Solutions A and B and co...
example 2
[0043] Granulation was carried out to obtain the nifedipine containing preparation in the same manner as that in Example 1, except that the aqueous solution of the water-soluble polymer was made by dissolving 20 g of hydroxypropylmethylcellulose (TC-5R: supplied by Shin-Etsu Chemical Co. Ltd.) in 180 g of purified water to render Spray B solution.
example 3
[0044] Granulation was carried out to obtain the nifedipine containing preparation in the same manner as that in Example 1, except that 200 g of lactose in Example 1 were replaced with the mixed powder of 170 g of lactose and 30 g of Hydrated Silicon-Dioxide (Carplex #80: supplied by Shionogi & Co. Ltd.).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com