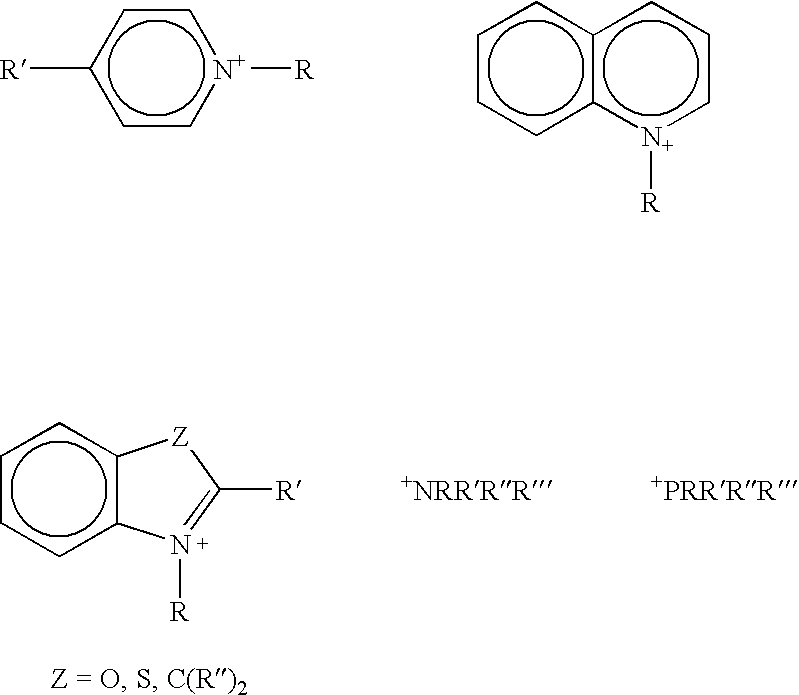
Curing agents for cationically curable compositions
a technology of cationically curable compositions and curing agents, which is applied in the field of curing agents for cationically curable compositions, can solve the problems of system imparting significantly reduced thermal stability to the cured material, high level of unreacted epoxy monomer present, and high level of unreacted epoxy monomer, so as to improve the dark stability, prolong the dark stability, and improve the effect of dark stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Properties of Comparative Formulations (No Added Salts)
Curable compositions were prepared using the general sample preparation method described above. DMA molds were used and the samples were given a total of 4 J / cm2 dose using the 600 W Fusion D system and a post cure as described in the sample preparation section. The amounts of material used and the results of the DMA, IR and visual color evaluation tests are shown in Comparative formulations Table 2, below.
TABLE 2Properties of Comparative Formulations (no added salts) Exposed to 4 J / cm2ComparativeUVI-UVI-ToneERL-Tg,Cure EstimateSample6990697402014221Color° C.from IRA0.4091 gNone4 g16 gcolorless 51 68%B0.3119 g0.1020 g4 g16 glight136100%brownC0.2562 g0.1531 g4 g16 glight124100%brownD0.2014 g0.2183 g4 g16 glight120100%brownE0.1528 g0.2622 g4 g16 glight119100%brownF0.1059 g0.3210 g4 g16 glight122100%brownG0.0497 g0.3608 g4 g16 glight125100%brownHnone0.4214 g4 g16 glight120100%brown
The Comparative Samples of Table 2 show that i...
example 2
Evaluation of Properties of Comparative Formulations (No Added Salts) vs UV Exposure
A stock solution of 36 g Tone 0201, 144 g ERL-4221 was mixed in a glass jar and placed in the oven at 100° C. for about 30 minutes to mix thoroughly before using. Individual curable composition samples were prepared by weighing out the desired amount of stock solution, UVI-6990 and UVI-6974 (Table 3) into a brown jar, heating the jars in an oven at 100° C. and mixing thoroughly. The compositions were allowed to cool to room temperature before proceeding. The amount used are described in Table 3, below.
TABLE 3Comparative Formulations (no addedsalts) For UV Intensity TestingComparativeStock Solution,SampleUVI-6990 UVI-697420% Tone 0201 / 80% ERL-4221I0.41 gNone20 gJ0.31 g0.10 g20 gK0.25 g0.18 g20 gL0.20 g0.22 g20 gM0.15 g0.25 g20 gN0.11 g0.31 g20 gO0.05 g0.35 g20 gPNone0.40 g20 g
The compositions in Table 3, above, were cured using the 600 W Fusion D (at 12.6 m / min) (548 J / pass) using the small sampl...
example 3
Addition of a Non-Photochemically Active Salt Containing an Inhibiting Anion to a Formulation with a Photochemically Active Salt Containing an Accelerating Anion
Individual curable composition samples were prepared by weighing out the amounts of TBA+ PF6−, ERL-4221E and UVI-6990 or UVI-6974 into a brown jar as shown in Table 6 below, heating the jars in an oven at 100° C. and mixing thoroughly. The compositions were allowed to cool to room temperature before proceeding.
TABLE 6Formulations With a Photochemically Active SaltContaining an Accelerating Anion with a Non-photochemically Active Salt Containing an Inhibiting Anion(Photocurative A)SampleAdditiveCatalystEpoxyQNone0.4 g UVI-699020.0 g ERL-4221E10.12 g TBA+ PF6−0.4 g UVI-697420.0 g ERL-4221E20.10 g TBA+ PF6−0.4 g UVI-697420.0 g ERL-4221E30.08 g TBA+ PF6−0.4 g UVI-697420.0 g ERL-4221E40.06 g TBA+ PF6−0.4 g UVI-697420.0 g ERL-4221E50.04 g TBA+ PF6−0.4 g UVI-697420.0 g ERL-4221ERNone0.4 g UVI-697420.0 g ERL-4221E
DMA molds were...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com