Method for producing cadaverine dicarboxylate
a technology of cadaverine and dicarboxylate, which is applied in the field of cadaverine dicarboxylate production, can solve the problems of inability to meet high temperature use, inconvenient production methods of biomass, complex process, etc., and achieve the effect of simple and efficient production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of a LDC-Amplified Strain of Escherichia coli
[0065] PCR primers having the nucleotide sequences of SEQ ID NOS: 1 and 2 were designed based on the nucleotide sequence of the LDC gene (cadA) of E. coli (Watson N. et al., Journal of Bacteriology, Vol. 174, 530-540, 1992; Meng S. Y. and Bennet G. N., Journal of Bacteriology, Vol. 174, 2659-2669, 1992) and used for PCR with the chromosome of E. coli W3110 (ATCC 39936) as a template to amplify a DNA fragment containing the cada gene.
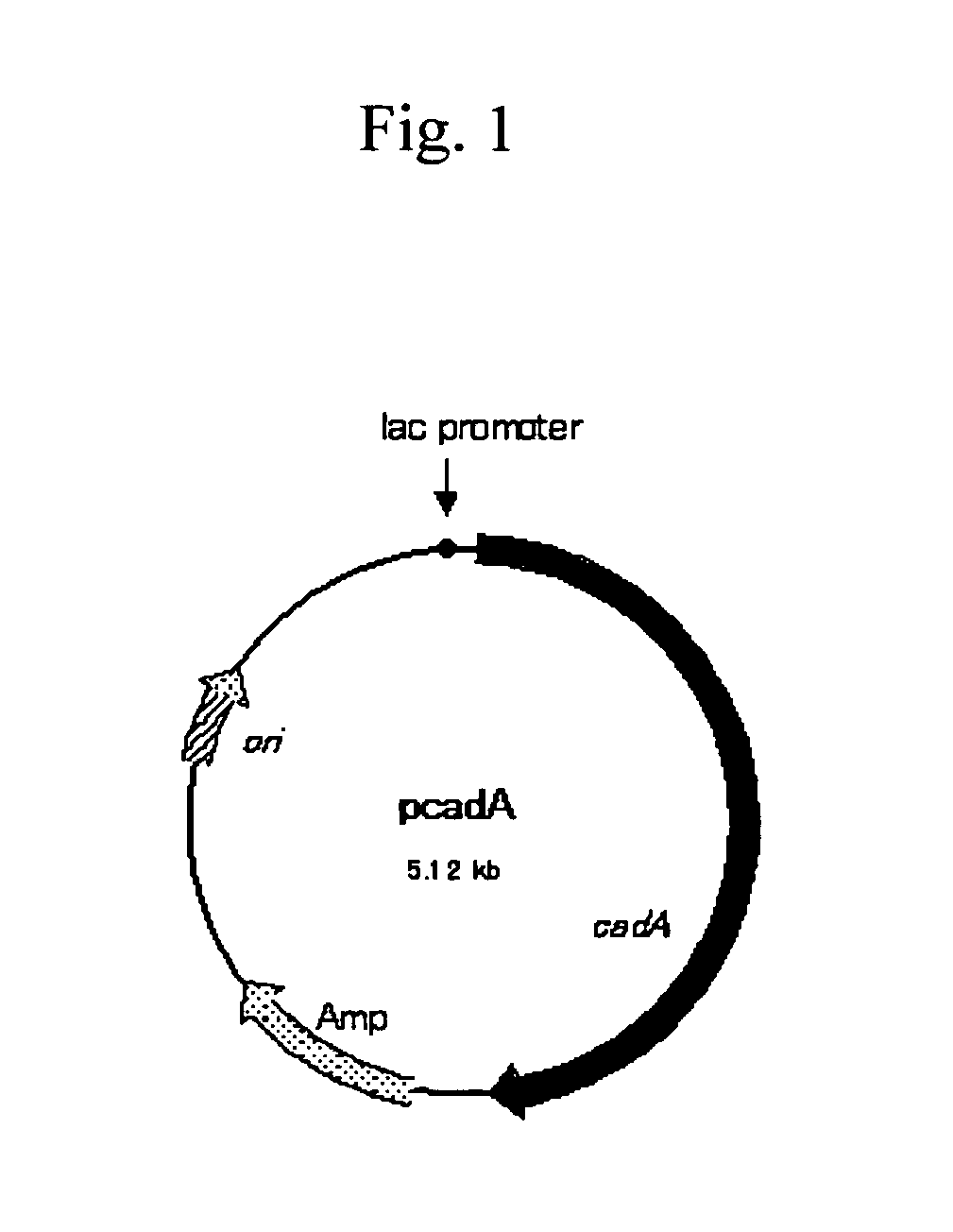
[0066] To prepare the pcadA plasmid, the amplified DNA fragment was digested with KpnI and SphI, and the resulting fragment (2468 bp) was inserted into the KpnI-SphI digestion site of pUC18 (Takara Shuzo) (FIG. 1). The E. coli JM109 strain (Takara Shuzo) was transformed with the pcadA plasmid. A transformant was selected using ampicillin resistance as a marker, and designated E. coli JM109 / pcadA.
example 2
Production of Cadaverine Adipate from Lysine Adipate Using cadA-Amplified Strain
[0067] (1) Culture of cadA-Amplified Strain
[0068]E. coli JM109 / pcadA was precultured in the LB medium, and then 50 ml of the culture broth was inoculated into 500 ml of the LB medium of two-fold concentration (2% of trypton, 1% of yeast extract, 1% of NaCl) contained in a 1-L jar fermenter (ABLE Co., Ltd.). The cells were cultured with aeration and stirring under the following conditions: aeration rate of 250 ml / min, 35° C. and 700 rpm. Following a 15 hour culture, the whole culture broth was inoculated into 22 L of LB medium of two-fold concentration contained in a 50-L jar fermenter, and the culture was continued under the following conditions: aeration rate of 11 L / min, 35° C., an internal pressure in the jar of 50 kPa and 250 rpm. Following the 4 hour culture, 3 g of IPTG (isopropyl-β-D-thiogalactopyranoside) was dissolved in 50 ml of water and added to the culture through a filter. Then, the cultu...
example 3
Acquisition of Cadaverine Adipate Crystals
[0075] (1) Removal of cells from cadaverine adipate solution
[0076] The cadaverine adipate solution obtained in Example 2 was sterilized in an autoclave at 120° C. for 10 minutes and centrifuged to collect a supernatant.
[0077] (2) Decoloration and Concentration
[0078] The obtained supernatant was added to 20% activated carbon based on the cadaverine weight and decolored with stirring at 20° C. for 1 hour. The activated carbon was removed using filter paper, and the obtained filtrate was concentrated 4- to 5-fold under reduced pressure (55 to 60° C., 110 to 150 mmHg). The solid content of the concentrate was 70 to 77%.
[0079] (3) Crystallization of Cadaverine Adipate and Separation of Crystals
[0080] The aforementioned concentrate was cooled from 60° C. to 10° C. at 4° C. / hour to precipitate crystals. The crystallization rate was 40 to 45%. The precipitated crystals were separated and collected using a centrifuge and air-dried in a desiccat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com