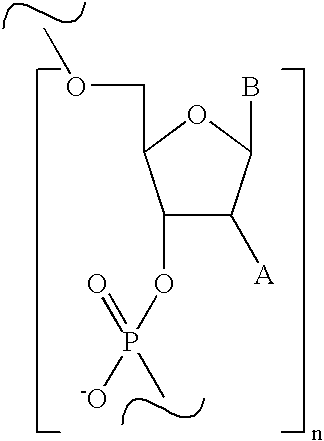
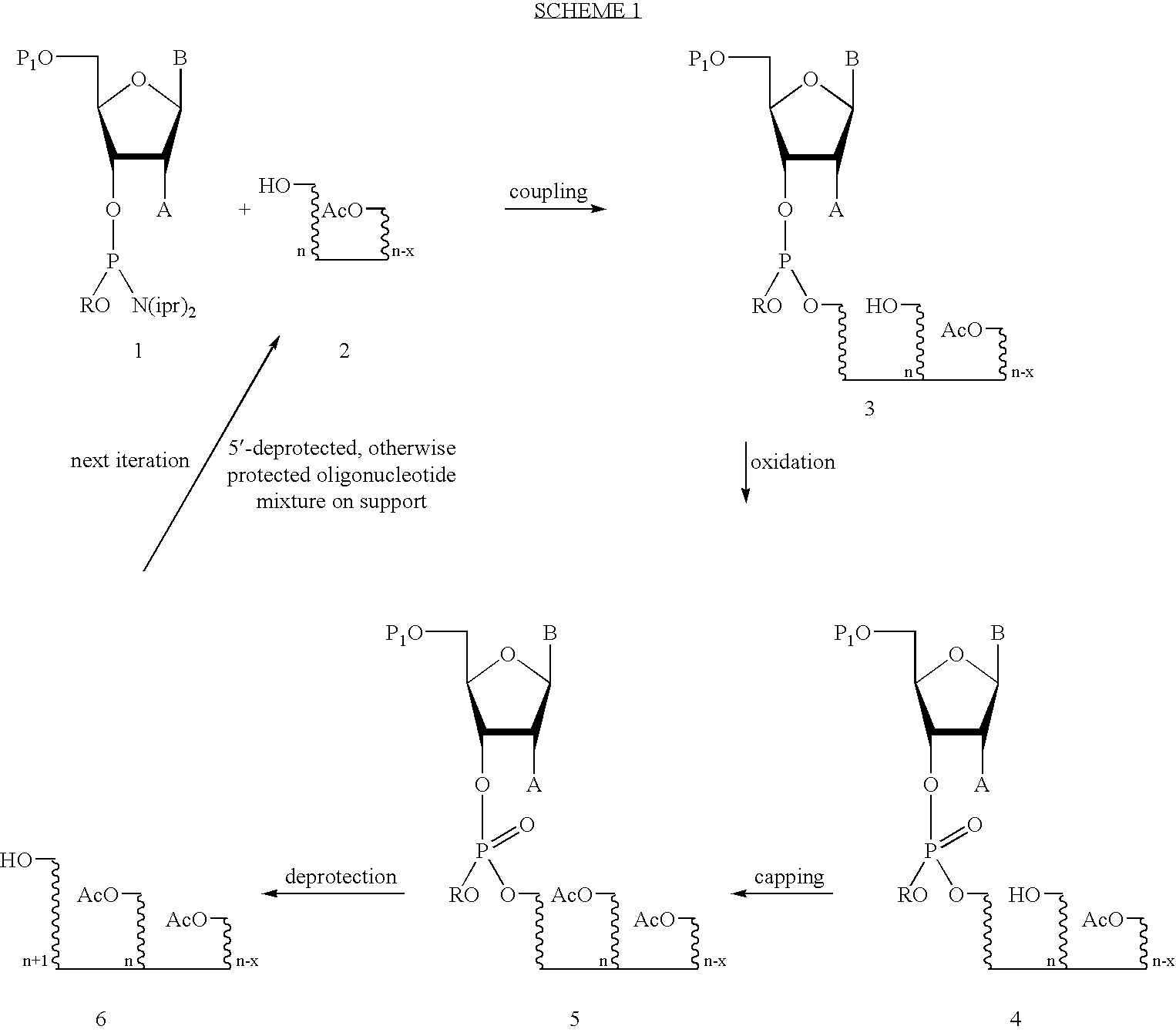Method for solution phase synthesis of oligonucleotides
a technology of oligonucleotide and solution phase, which is applied in the field of nucleic acid chemistry, can solve the problems of failure sequences, oligonucleotides that have not been extended by one nucleotide monomer, and little attention has been paid to the preparation and isolation of these compounds on a scale that allows clinical development, etc., and achieves the effects of easy analysis, low cost and low cost of remaining materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 3 (
[0085] Example 3 (Scheme 6) describes the synthesis of a phosphoramidite monomer containing 5'-O-(4,4'-dioctadecyloxytrityl) (DOT) as the 5'-protecting group (D-E).
[0086] Example 4 illustrates the ability to separate the coupling product from the unreacted oligonucleotide starting material (failure sequence) based upon the selective or specific interaction of the 5'-protecting group (D-E) with a particular resin or phase. In this example, the mobility of 4,4'-dioctadecyltriphenylmethanol (DOT) 23 on a C18 reverse phase resin is compared to that of 4-decyloxy-4'-methoxytritanol and dimethoxytritanol (DMT) (see Table 1). The strong interaction of the DOT group with C18 resin in organic solvents, such as methanol (R.sub.f=0) and acetonitrile (R.sub.f=0) enables the one-step separation of product from starting material by loading the mixture onto C18 resin and washing the unreacted starting material away with an organic solvent. The coupled product can then be eluted from the chamber by...
example 12 (
[0100] Example 12 (Scheme 15) describes the preparation of a dimer using product capture by Diels-Alder cycloaddition. The rate of capture of the 3',3'-linked 5'-DHDTO-T-T dimer is dependent on the excess of resin bound maleimide groups. Product capture proceeds quantitatively. The captured product is easily and quantitatively released from the resin with 3% dichloroacetic acid in dichloromethane. After neutralization and concentration, pure product is obtained.
[0101] Example 13 describes a method for assembly of oligonucleotides from blocks by capturing one of the blocks on a resin using the cycloaddition of a 5'-O-(4,4'-di-3,5-hexadienoxytrityl) protected oligonucleotide to a dienophile derivatized resin.
[0102] Example 14 (FIG. 8) illustrates schematically an automated extraction / filtration system 200 and process designed for the automated preparation of an oligonucleotide bearing a 3'-terminal polyethylene glycol using covalent capture of the monomer addition product at every cyc...
example 1
Preparation of N-4-benzoyl-3'-(5'-tert-butyldimethylsilyl-3'-(2-cyanophosp- horyl)thymidyl)-2'-fluorocytidine (16) (Scheme 4)
[0110] 5'-tert-butyldimethylsilylthymidine 12 (5'-TBDMS-thymidine) (0.15 g. 0.42 mmol) was dissolved in dry acetonitrile (10 mL) under an argon atmosphere. Cytidine amidite 13 was added (0.43 g, 0.50 mmol) followed by tetrazole (6.5 mL. 0.45 M in acetonitrile). After 15 minutes reverse phase HPLC analysis (C18, 4.6.times.100 mm, Buffer A: 100 mM triethylamrnmonium acetate pH 7.5. Buffer B: acetonitrile, 0 to 80% B over 2.5 minutes) of the reaction mixture showed the presence of dimer (2.4 minutes) as well as unreacted thymidine 12 (1.4 minutes) and hydrolyzed amidite monomer (2.1 minutes) (FIG. 1). The reaction mixture was oxidized in situ (10 mL, 0.2 M iodine in water / pyridine). HPLC analysis after oxidation reveals the presence of pyridine (0.9 minutes), unreacted thymidine 12 (1.4 minutes), oxidized amidite monomer 15 (1.8 minutes), and oxidized dimer 14 (2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com