[0011] The
advantage of the invention is that the any escape of material from the electrochemical cell is prevented, with the use of a sealing ring, by the capillary action of the tip and of the container, and that the surface is wetted with a defined electrolyte simply by removing the cap and applying the tip. Since both the subsequent flow of the electrolyte to the surface and from the container to the tip is achieved exclusively by capillary action, the function of the electrochemical cell is completely independent of the effect of gravity. The result is that measurements can also be made on surfaces vertically oriented surfaces. In addition, it is easily possible to perform measurements in
zero gravity. Since the container is open,
atmospheric pressure also has no significant effect on the performance of measurements.
[0012] Since the cross-section of the tip is tapered toward the end and the container has maximum
porosity, a large volume is available to conduct the electrolytic current in the container and in the tip. The ohmic
voltage drop thus occurs only at the very end of the tip. As a result, any
distortion of the measurement by the ohmic
voltage drop can be minimized.
[0014] The use of a silver surface coated with
silver chloride, or a silver surface, or a
tungsten surface creates a very simple reference
electrode which remains stable and completely maintenance-free for months at a time. There is no possibility of
contamination of the electrolyte, such as occurs with most reference electrodes, by the saturated
potassium chloride solution. The result is that the complete cell along with the electrolyte may be prefabricated. Operation is extremely simple and does not require a
technician. No manipulation of the electrolyte is required. The entire procedure consists in the removal of the cap from the electrochemical cell and application to the examination surface. After use, the cap is replaced and the electrochemical cell is ready for the next use.
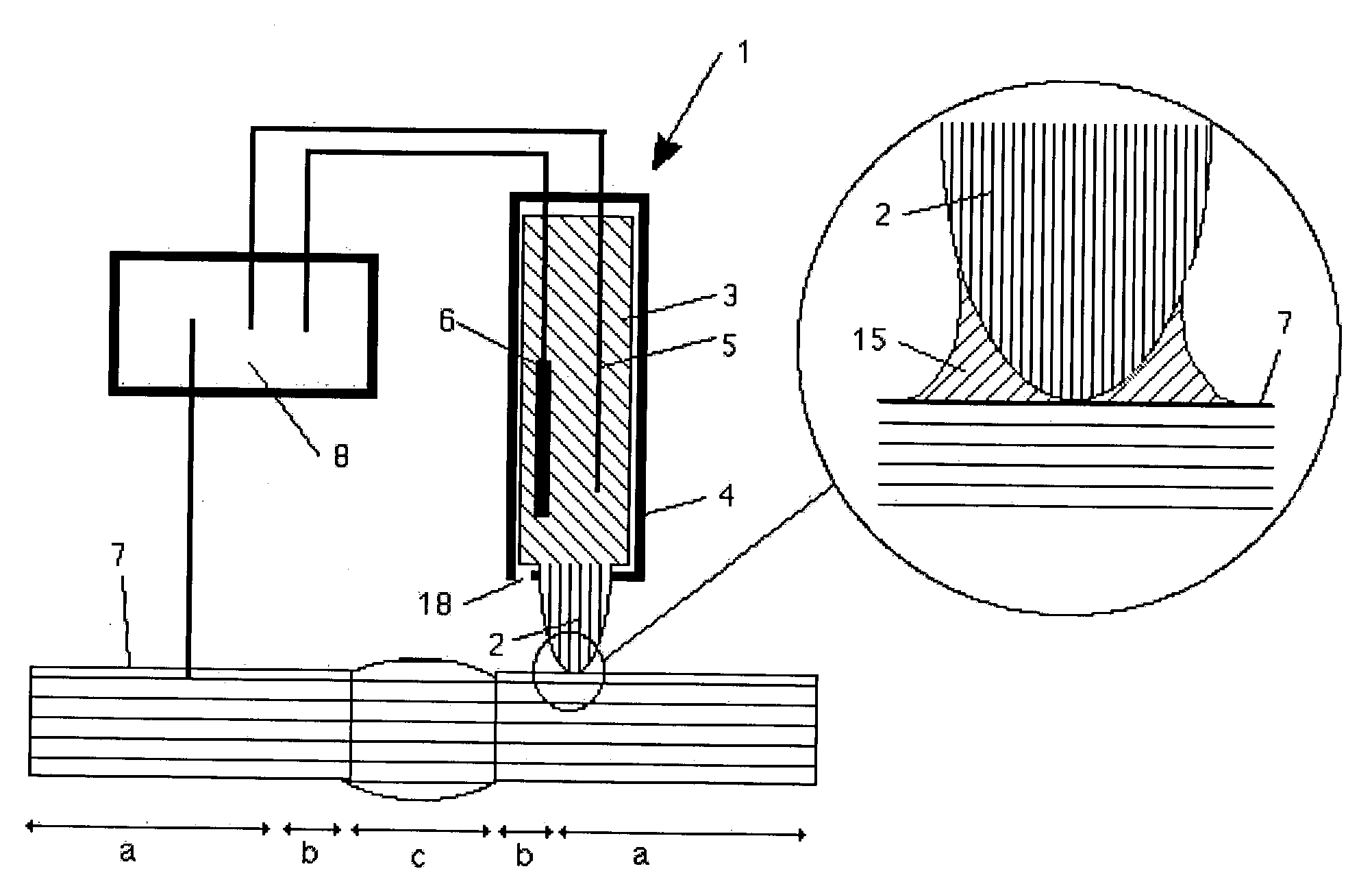
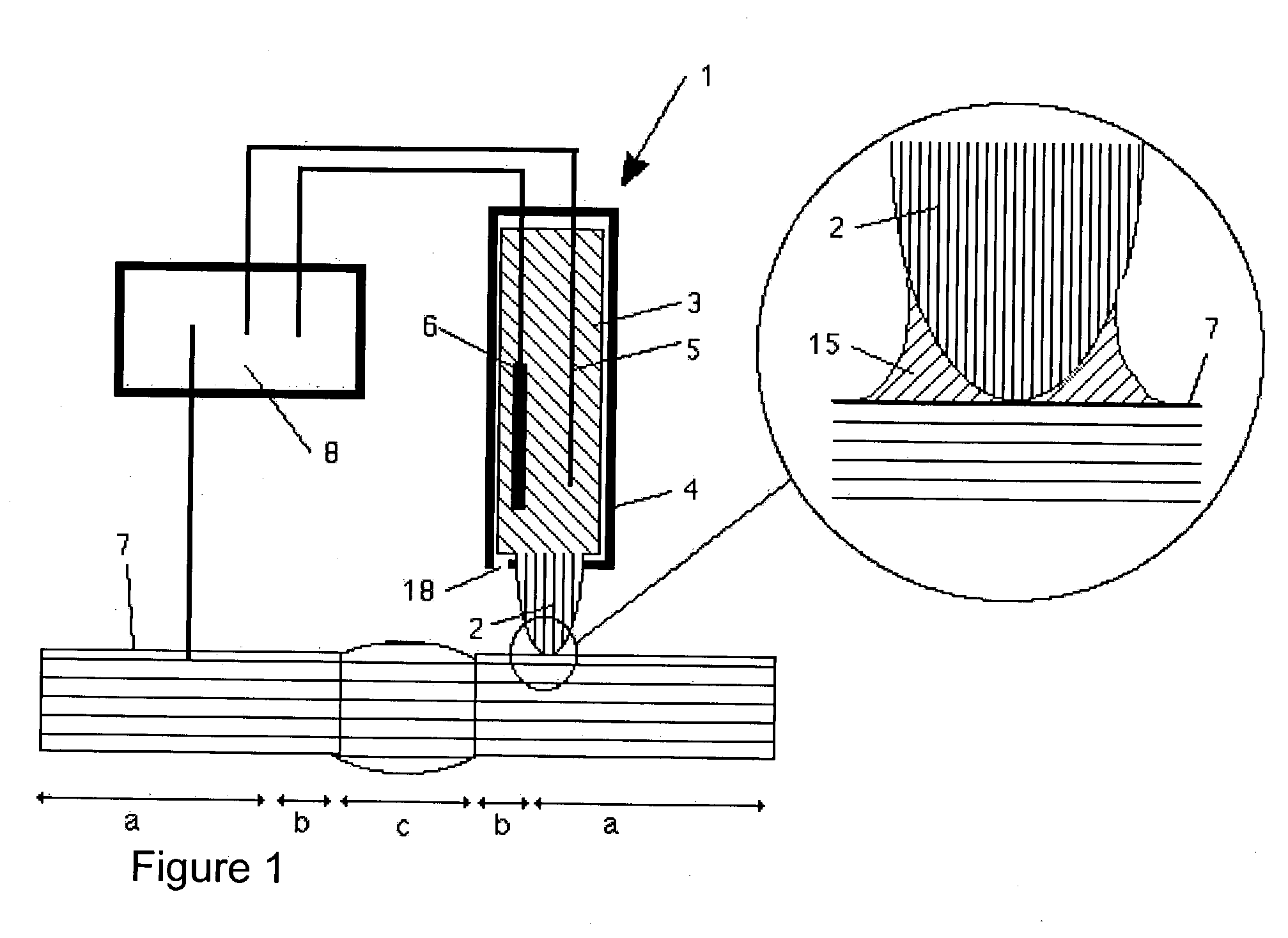
[0015] Since any escape of material from the electrochemical cell is prevented by the capillary action of the tip even without the tip's contacting the surface, the electrochemical cell is extremely simple to manipulate. It may, for example, be held in the user's hand and applied to the highly curved surface of any large component (computer
chip, automobile, pipeline, etc.). Since the interface of the tip is only at a
single point, or the tip conforms elastically to the surface, any type of curved surface geometries may be examined. When a sealing ring is used, the surface must allow for a circular interface of the sealing ring, a fact which results in certain requirements in terms of flatness or surface
radius. The surface of the component is wetted locally with electrolyte, thus allowing electrochemical investigations of local resolution. This type of simple operation was previously impossible using conventional electrochemical cells for large components with complex geometries and vertically oriented surfaces. With the invention, for example, the quality of a
welding seam on a pipeline may be easily inspected without having to
cut out the
welding seam,
grinding it flat, and inserting it into a classic cell. Thanks to the invention, it is therefore possible to conduct electrochemical investigations in a standardized routine fashion as a nondestructive test method in
quality assurance, research, etc. Since no measures related to sealing, such as sealing rings, are required, the tip may be moved continuously along the surface, thereby allowing for simple electrochemical inspection of large surfaces with local resolution. Multiple sequential spot measurements are also easily performed. The electrochemical cell may be employed for a multiplicity of electrochemical investigations and processes. After completion of the electrochemical modification, the electrochemical cell is simply lifted from the surface and closed. When lifted, any escape of material from the cell is prevented by the capillary action of the tip. The electrochemical cell may be stored for long periods in a closed condition and used at any time without any preparatory efforts. This means a great savings in time when conducting electrochemical measurements. In addition, the electrochemical cell may be commercially prefabricated complete and
ready to use, thus enabling its use in a standardized routine manner.
[0016] During prolonged measurements, the electrolyte is able to evaporate at the tip, thus leading to an increase in concentration. This may be prevented by surrounding the tip by a jacket. A high level of
humidity is quickly established within the jacket which prevents any further
evaporation. As a result, prolonged measurements may be performed with the electrochemical cell. The jacket may also be provided with additional functions. It may, for example, be composed of a conductive material, thereby creating an electromagnetic shield for the cell or the conductive contact with the surface. By additionally utilizing a spring, the jacket may also be used to ensure a constant application pressure. This feature enhances the reproducibility of measurements and the useful life of the tip.
[0017] Due to its porous structure, the tip prevents any
convection of the electrolyte. For processes which are controlled by
mass transfer, such
convection produces poor reproducibility and prevents meaningful findings. In the tip, the subsequent transfer of the initial constituents for the electrochemical reactions is controlled almost exclusively by
diffusion. This means that reproducible results are obtained. Use of the invention enables characterization of the
mass transfer processes to be significantly improved.
 Login to View More
Login to View More