Neutralized monoclone antibody 4C13 of antiricin, its production and use
A monoclonal antibody, ricin technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, antibodies, etc., can solve the problems of simultaneous recognition of ricin monoclonal antibodies, etc., and achieve broad application Foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The construction of embodiment one anti-ricin monoclonal antibody hybridoma cell line
[0035] 1. Materials:
[0036] Freund's complete adjuvant and incomplete adjuvant, TMB is a product of Sigma, 20% fetal bovine serum is a product of Beijing Yuanheng Shengma Biotechnology Research Institute, serum-free RPMI 1640 is a product of Gibco, and SP2 / 0 cells are imported from ATCC , preserved in our laboratory, and Balb / c mice and Kunming mice were purchased from the Experimental Animal Center of the Academy of Military Medical Sciences. The rest of the reagents were purchased from the market.
[0037] 2. Method results:
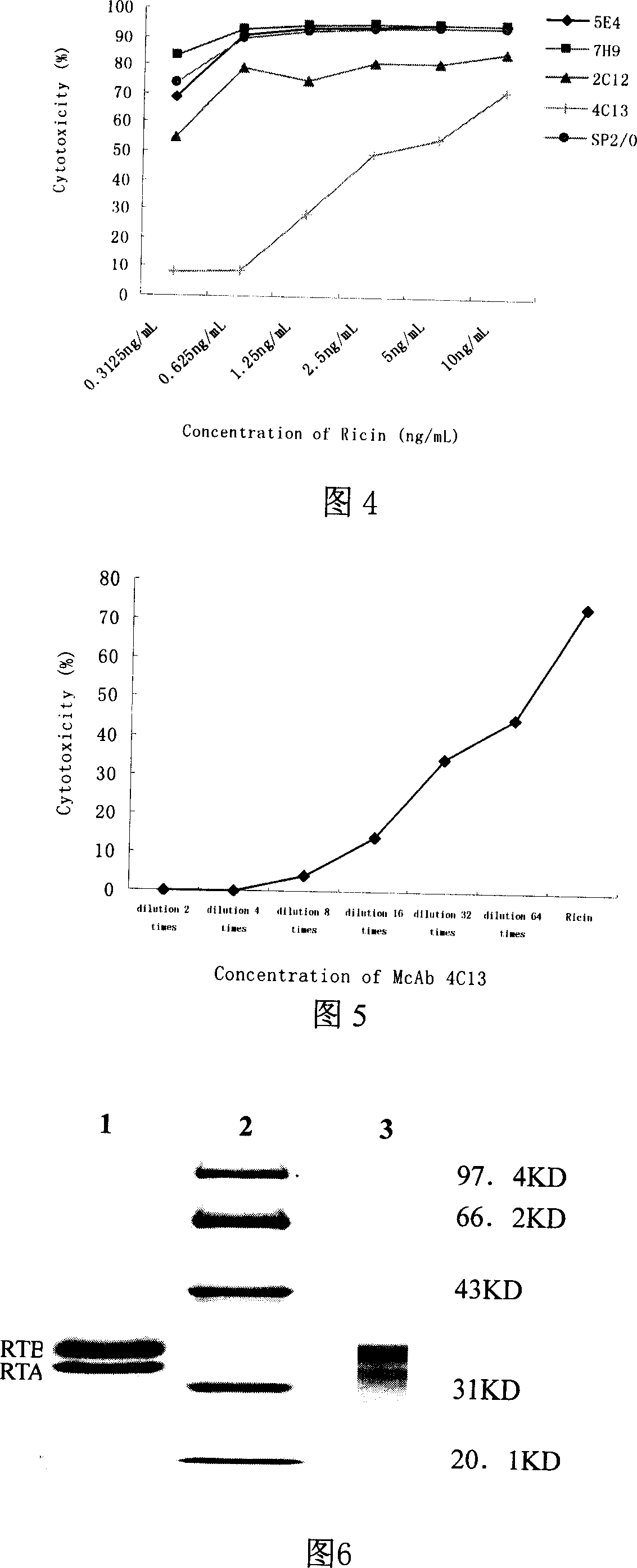
[0038] 1. The attenuation treatment of ricinus in the purified ricin (Nicolson, G.L., Blaustein, J., 1972.The interaction of Ricinus com-munis agglutinin with normal and tumor cell surfaces.Biochim.Biophys.Acta 266,543 Formaldehyde with a final concentration of 1% was added to -547.) 2mg / ml, treated at 37°C for 72 hours, dialyzed in PH7.20.01M PBS for 72...
Embodiment 2
[0046] Example 2 Screening and identification of anti-ricin neutralizing monoclonal antibody hybridoma cell line
[0047] 1. Materials:
[0048] Ditto.
[0049] 2. Method results:
[0050] 1. Determination of the median lethal dose (IC50) of ricin on mouse myeloma cell SP2 / 0 Dilute ricin with RPMI-1640 so that the concentrations are 0ng / mL, 20ng / mL, 10ng / mL, 5ng / mL, 2.5ng / mL mL, 1.25ng / mL, 0.625ng / mL, 0.3125ng / mL, 0.156ng / mL, 0.078ng / mL were added to 96-well cell culture plate respectively, each concentration was replicated in 3 wells, 100μl / well, logarithmic adjustment The concentration of SP2 / 0 cells in the growth phase was adjusted to 5 × 10 5 / ml, 100 μl / well. Cultivate in a 5% CO2 incubator at 37°C for 24 hours, add 10 μl MTT to each well, incubate in a 5% CO2 incubator at 37°C for 6 hours, centrifuge at 2000 r / min for 10 minutes, discard the supernatant, add 100 μl DMSO to dissolve the crystals, and measure the A value at a wavelength of 570 nm . According to the fo...
Embodiment 3
[0056] The Protein A purification of embodiment 3 monoclonal antibody 4C13 and
[0057] Protective Experiments on BALB / C Mice
[0058] 1. Materials:
[0059] Protein A Sepharose CL 4B column protein column: product of Beijing Benyuan Zhengyang Biotechnology Co., Ltd.; same as above.
[0060] 2. Method results:
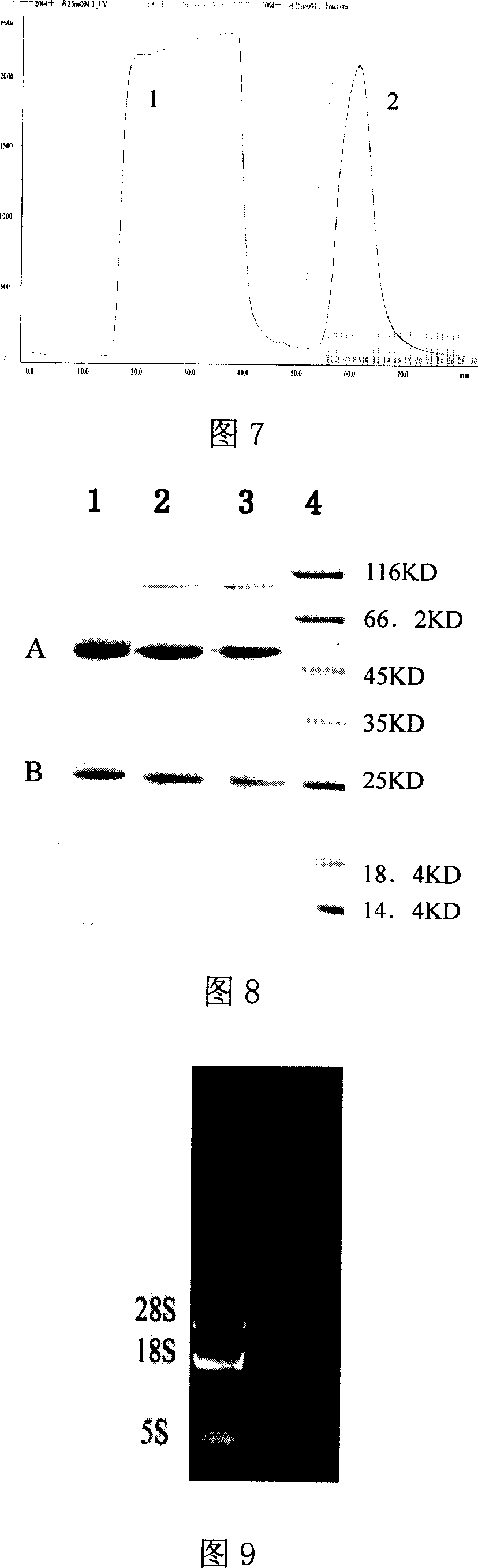
[0061] 1. Add 1 ml of pH8.0, 0.1moL / L phosphate buffer to 2 ml of mouse ascites and adjust the pH to 9 with pH9.0, 1moL / L TRIS-HCL. Add mouse ascites to the Protein A Sepharose CL 4B column protein column that has been equilibrated with 0.1moL / L phosphate buffer solution pH 8.0, and wash the column with the above buffer solution until no foreign protein is detected in the effluent. Elute with pH 3.0 citrate buffer, collect the effluent, and immediately neutralize with 1moL / L TRIS-HCL pH 8.5 buffer, and dialyze with pH 7.2, 0.01M PBS for 72h. Samples were taken to measure OD260 and OD280 on a UV spectrophotometer, the protein content was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com