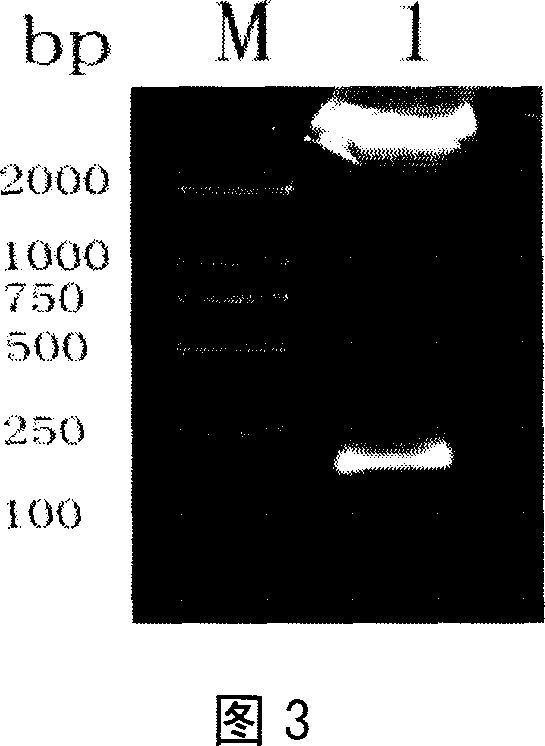Pig's epidermal growth factor gene and its application
An epidermal growth factor and gene technology, applied in the field of genetic engineering, can solve the problems of high price and difficult to meet production needs, and achieve the effects of low production cost and simple purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
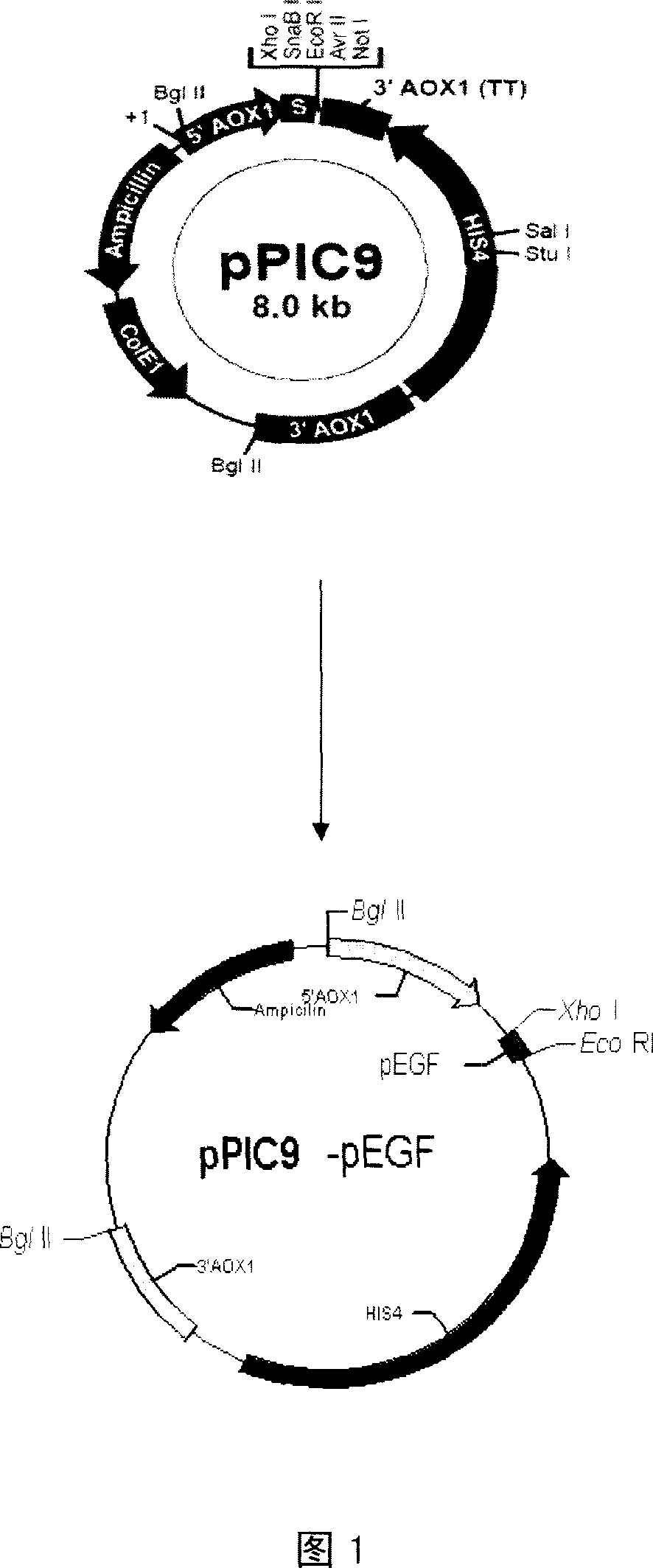
[0026] Example 1 Construction of expression vector
[0027] According to the amino acid sequence of pEGF and the codon preference of Pichia pastoris, the pEGF gene was artificially synthesized. Its nucleotide sequence and its coded amino acid sequence are respectively shown in SEQ ID NO.1 and 2 of the sequence listing. The above sequence was synthesized by Shanghai Yingjun Company. Using the synthetic pEGF gene as a template, design a pair of primers so that the 5' end of the upstream primer contains the sequence between Xho I and SnaB I on the pPIC9 vector, and the 5' end of the downstream primer contains 6 histidines, a TGA Stop codon and EcoR I site, the upstream primer used in this example is: 5'GCGCGCTCGAGAAAAGAGAGGCTGAAGCTAATTCTTACTCTGAATGTCCCAC, the downstream primer: 5'GGGCCGAATTCTCAATGATGATGATGATGATGTCTCAACTCCCACCATTTCAAG. PCR amplification was performed using this pair of primers. The PCR product and the pPIC9 vector were digested with Xho I and EcoR I respectivel...
Embodiment 2
[0031] Example 2. Preparation, transformation and screening of transformant phenotypes of competent host bacteria
[0032] Common solutions and media for yeast expression systems:
[0033] 10×D: Weigh 20g of D-glucose, add double distilled water to 100mL, heat until completely dissolved, filter or autoclave, store at 4°C, and the storage period is one year.
[0034] YPD medium (Yeast Extract Peotone Dextrose Medium): Weigh 10 g of yeast extract and 20 g of tryptone, dissolve in 900 mL of double distilled water, autoclave for 20 min, and then add 100 mL of sterilized 10×D.
[0035] 10×YNB (13.4% Yeast Nitrogen Base with Ammonium Sulfate without Amino Acid): Weigh 67g of Yeast Nitrogen Base (YNB), add double distilled water to 500mL, heat until completely dissolved, filter and sterilize, store at 4°C, shelf life for one year.
[0036] 500×B (0.02% D-Biotin, D-biotin): Weigh 10mg of D-biotin, add double distilled water to 50mL, heat until completely dissolved, filter and steril...
Embodiment 3
[0054] Example 3 Dot hybridization screening of multi-copy integrated transformants
[0055] Yeast lysis buffer: 0.01M Tris-Cl (pH7.6), 0.5M EDTA (pH8.0), β-mercaptoethanol (1% V / V).
[0056] Dot blot solution (1×): 20×SSC 250mL, 5% SDS 5mL, 10% Sarkosyl 10mL, ddH 2 O 735mL, Blocking Reagent 5g.
[0057] The phenotype-determined transformants were placed in a 96-well plate containing 100 μl of YPD medium per well, cultured at 30°C for 48 h, 10 μl was inoculated into a 96-well plate containing 100 μl of fresh YPD medium per well, and cultured for 48 h. Then take 50 μl of the cultured bacterial solution from each well in a 2ml centrifuge tube, centrifuge to remove the supernatant, resuspend the cell pellet with 80 μl of yeast lysis buffer containing yeast lyase (lyticase), lyse at 37°C for 4 hours, and then act at 100°C for 10 minutes , immediately put the centrifuge tube in ice, and add an equal volume of 20×SSC to obtain the lysate. Using a dot blot injector, transfer the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com