Aromatic enediyne derivatives, organic semiconductor thin films using the same and manufacturing methods thereof, and electronic devices incorporating such films
A technology of aromatic enediynes and derivatives, which is applied in semiconductor devices, semiconductor/solid-state device manufacturing, electric solid-state devices, etc., and can solve problems such as the high price of pentacene and the inapplicability of preparing organic semiconductor films.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
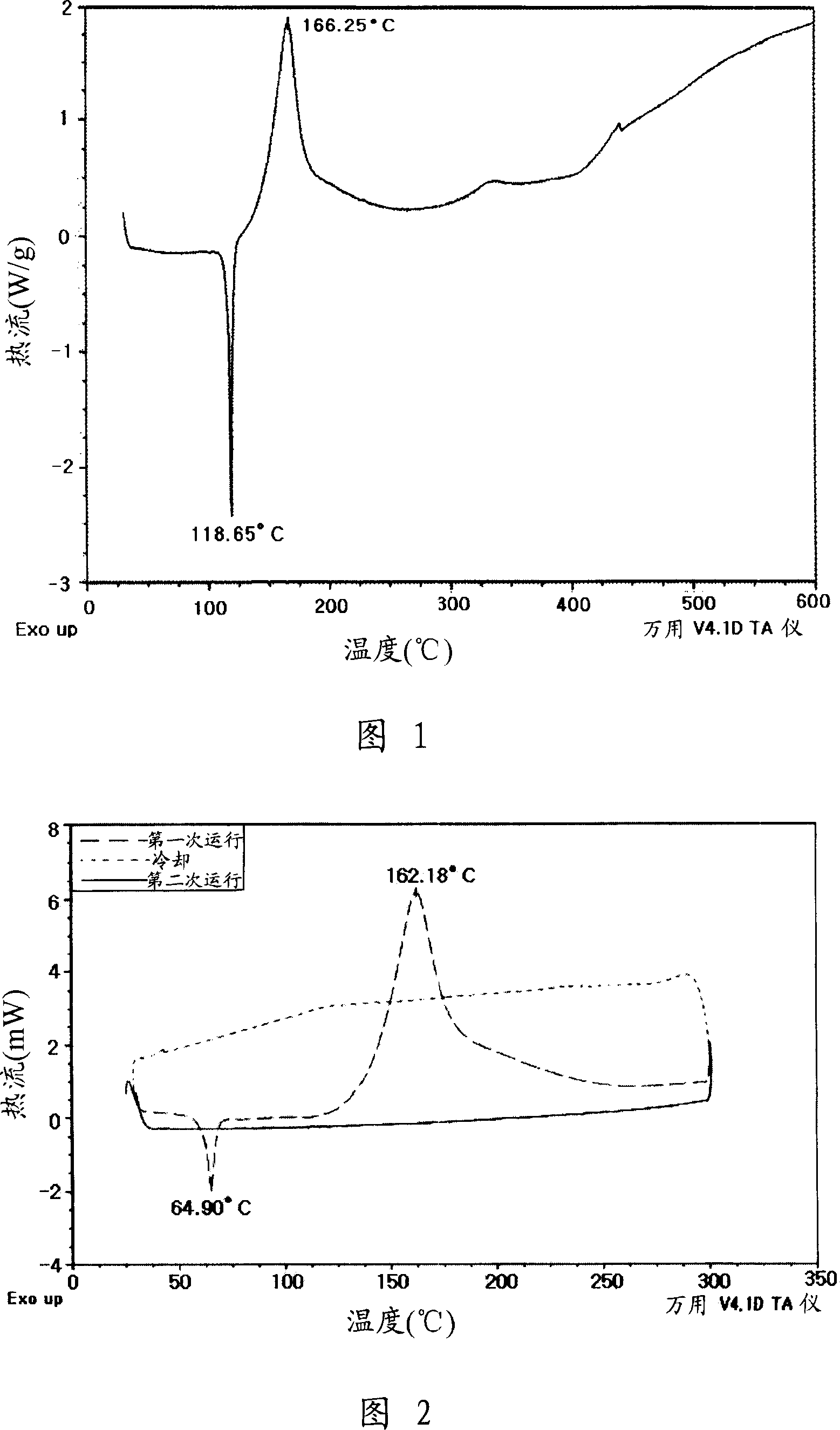
[0065] Preparation Example 1: Synthesis of Arenediyne Derivative A Example
[0066]
[0067] 2ml (18.0mmol) of 2,3-dibromothiophene and 3.5ml (27.0mmol) of 1-heptyne were mixed with tetrahydrofuran / diisopropylamine (1:1) solvent, and 0.23g (0.36mmol) of dichloro Diphosphine palladium, 70 mg (0.36 mmol) of copper iodide and 0.1 g (0.36 mmol) of triphenylphosphine were subsequently added thereto. The reaction solution was heated at 70°C for 8 hours, and then washed with an aqueous ammonium chloride solution. The obtained organic layer was dried over magnesium sulfate, dried under reduced pressure, and purified by silica gel column chromatography, thereby obtaining 4.6 g of 2-heptynyl-3-bromothiophene. 3.3 ml (23.2 mmol) of trimethylsilylacetylene was added to the compound obtained above, and then it was returned to the above synthesis process to prepare 2.7 g (9.84 mmol) of 2-heptynyl-3-trimethylmethyne The silylethynylthiophene was then mixed with 12.8 ml (12.8 mmol) lithi...
preparation Embodiment 2
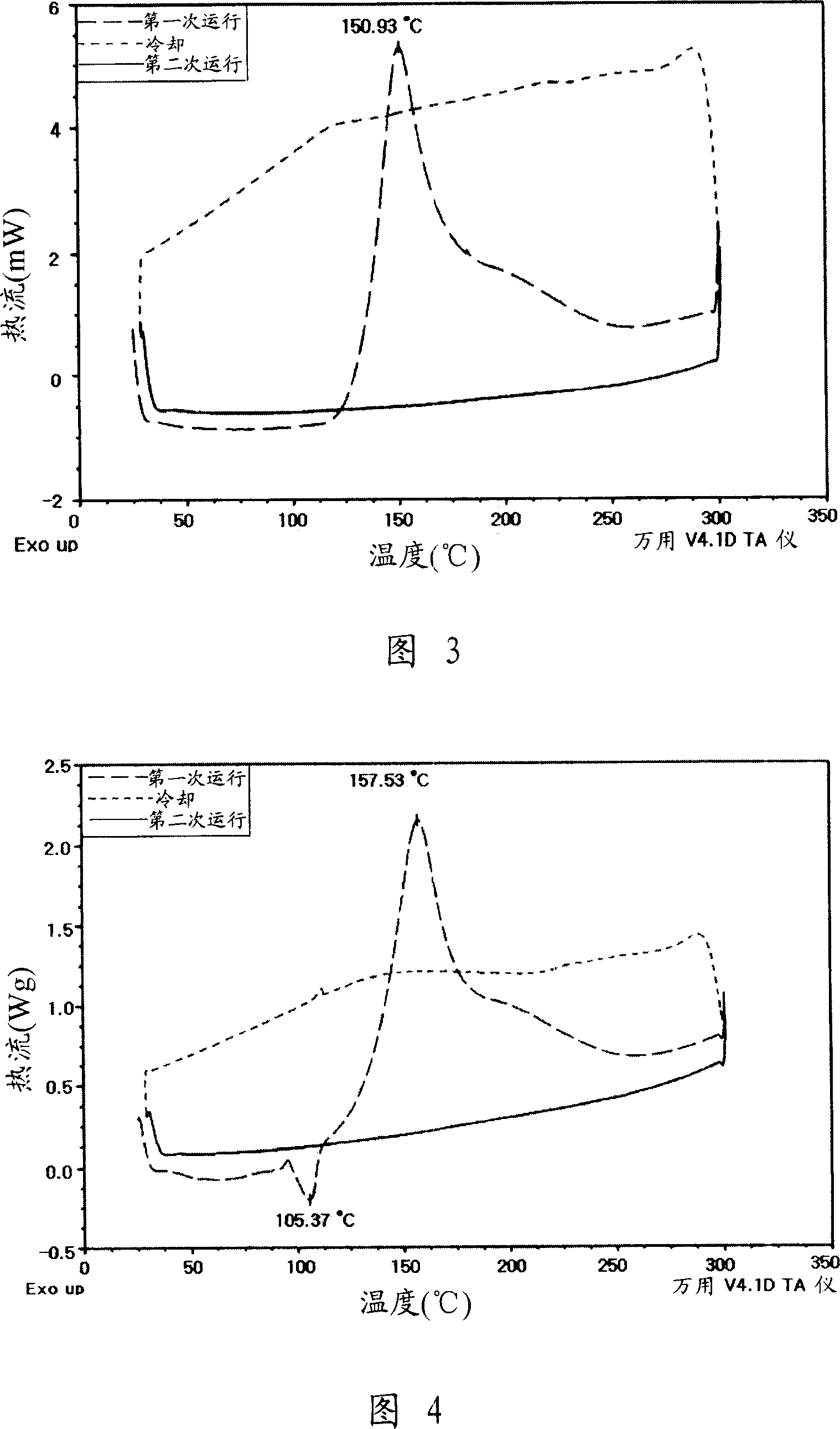
[0070] Preparation Example 2: Synthesis of Arenediyne Derivative B Example
[0071]
[0072] 0.25 g (1 mmol) of tetrathiophene (terthiophene) was added to chloroform, and 0.35 g (2.0 mmol) of N-bromosuccinimide was added thereto, thereby obtaining dibromide 1b, which was then mixed with synthetic derivatives A derivative B was obtained by Suzuki coupling and desilylation reaction under the same synthesis conditions. Analyzing the prepared derivative B obtains the following NMR data: 1 H NMR (300MHz, CDCl 3 ), δ(ppm) 0.93(t, 6H, J=7.2Hz), 1.24-1.67(m, 12H), 2.51(t, 4H, J=7.0Hz), 3.26(s, 2H), 7.05-7.08( m, 8H).
preparation Embodiment 3
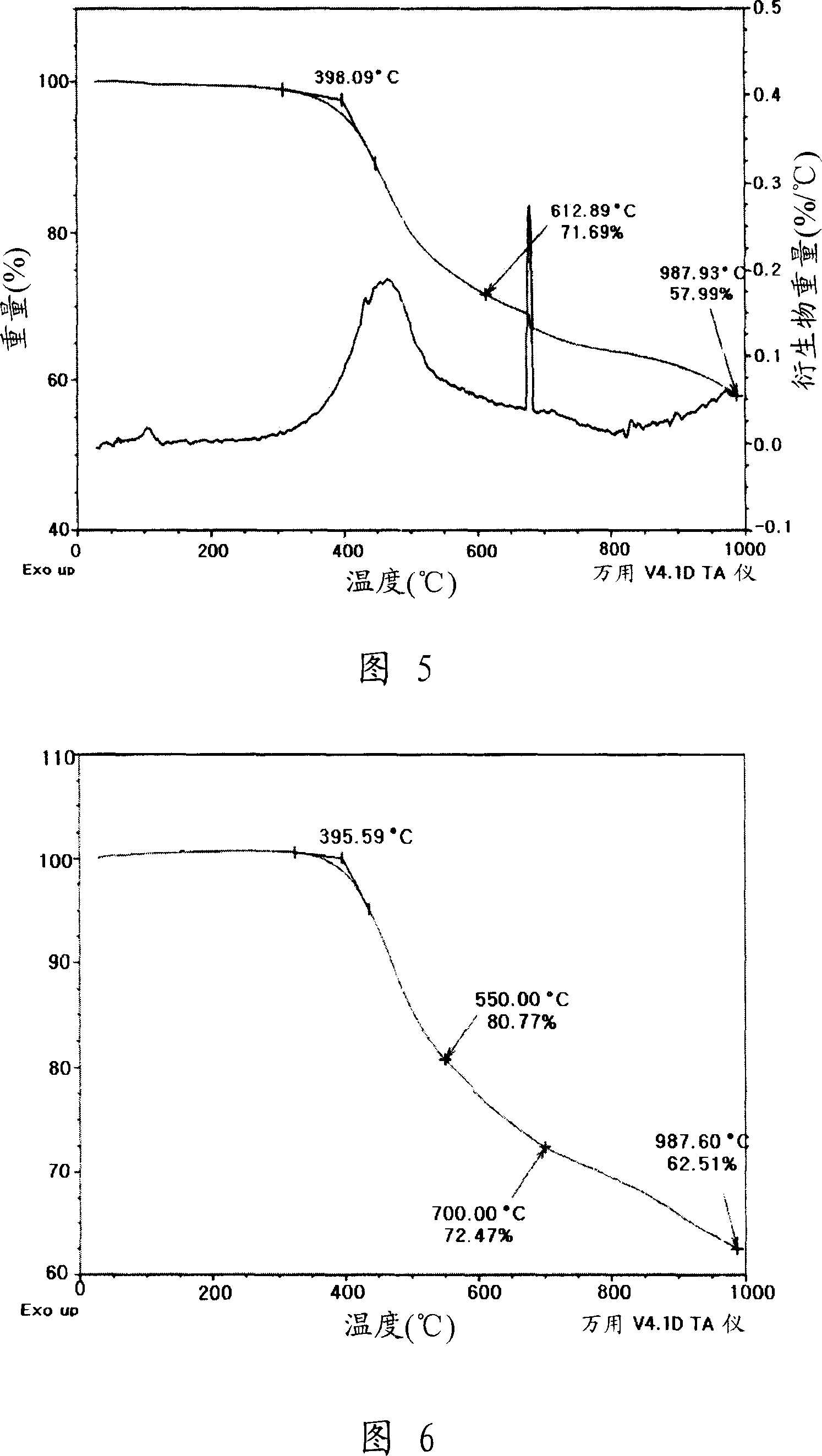
[0073] Preparation Example 3: Synthesis of Arenediyne Derivative C Example
[0074]
[0075] 2,2'-Dibromodithiophene and 2-bromothiophene are subjected to Suzuki coupling, thereby obtaining the intended or desired product, to which N-bromosuccinimide is then added to prepare dibromotetrathiophene 1c. Subsequently, the compound 1c was subjected to Suzuki coupling and desilylation reaction under the same synthesis conditions as for the synthesis of derivative A, whereby derivative C was obtained. The derivative C prepared by analysis obtains the following NMR data: 1 H NMR (300MHz, CDCl 3 ), δ(ppm) 0.93(t, 6H, J=7.2Hz), 1.24-1.67(m, 12H), 2.51(t, 4H, J=7.0Hz), 3.27(s, 2H), 7.05-7.09( m, 10H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com