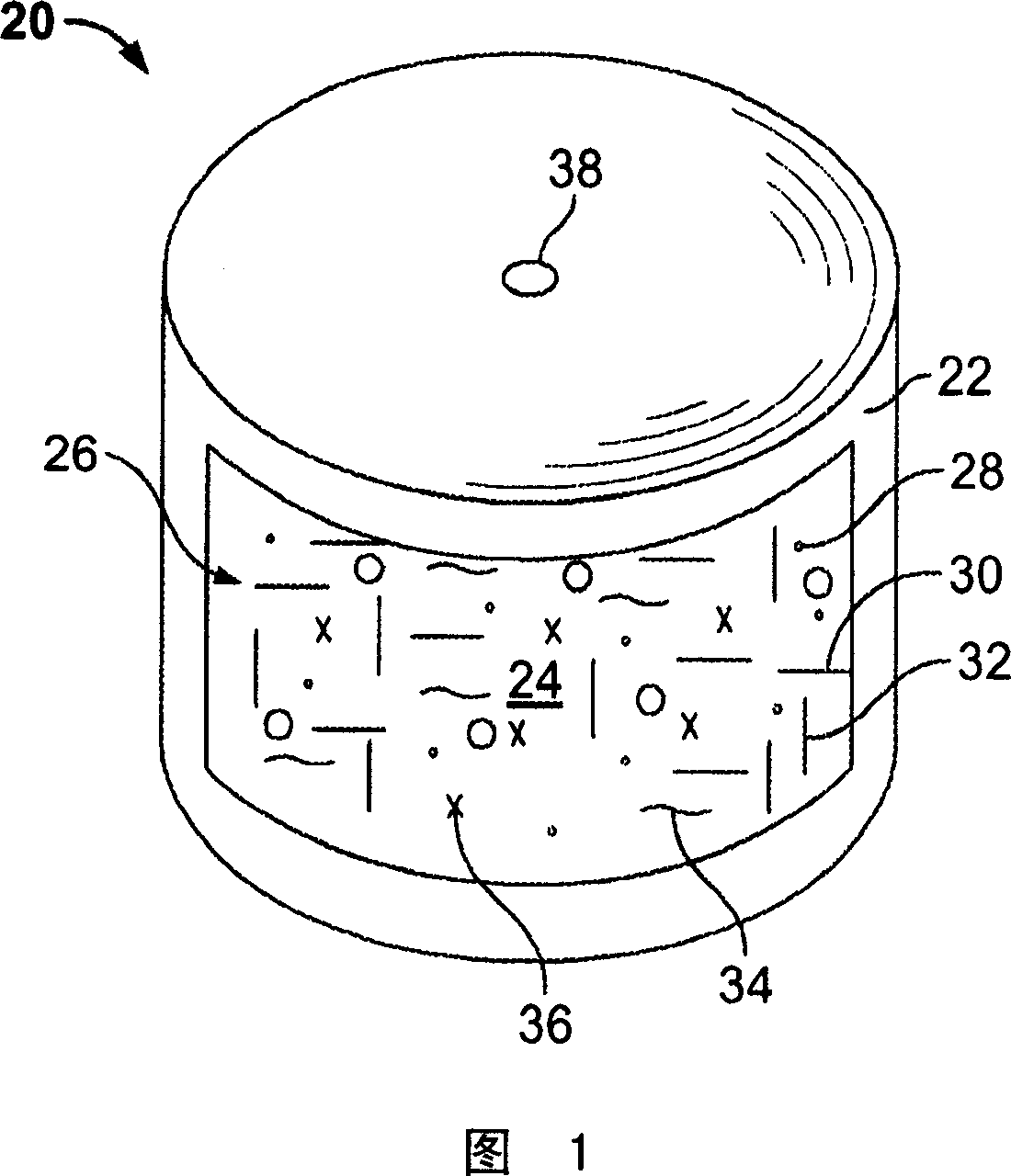
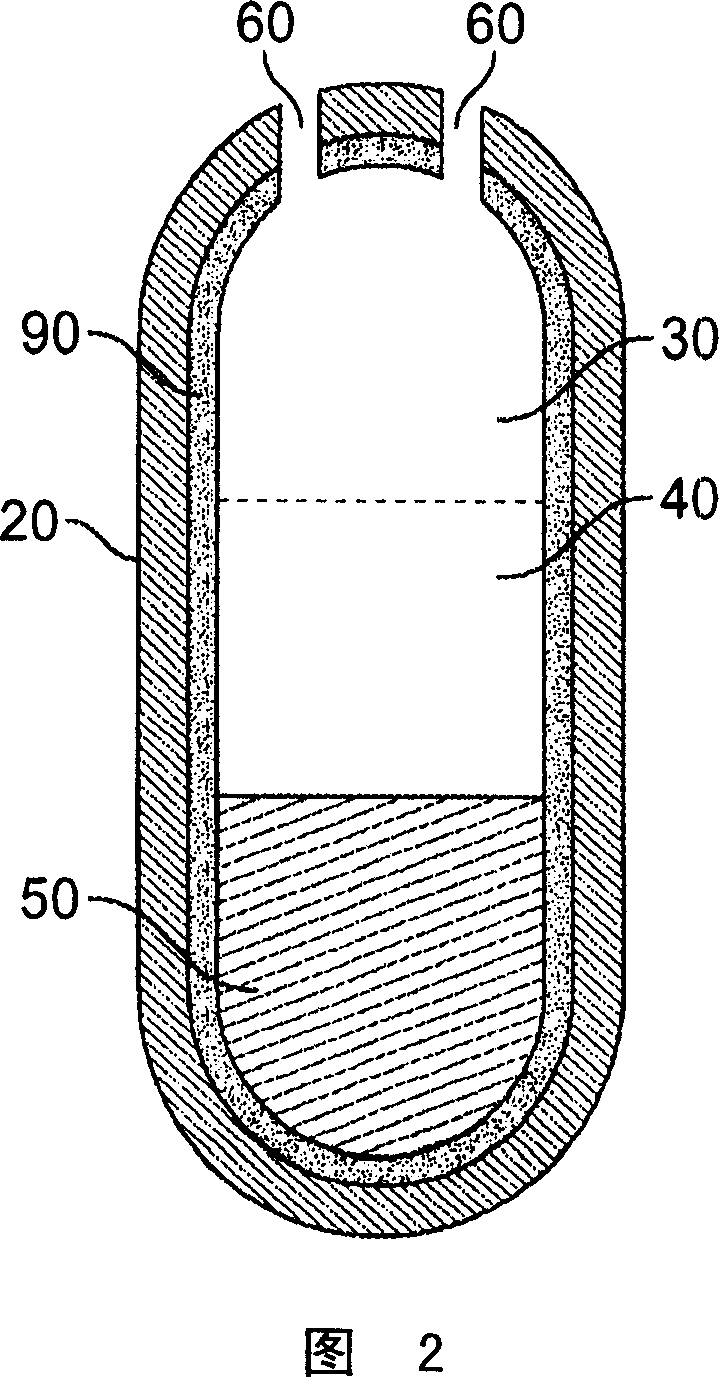
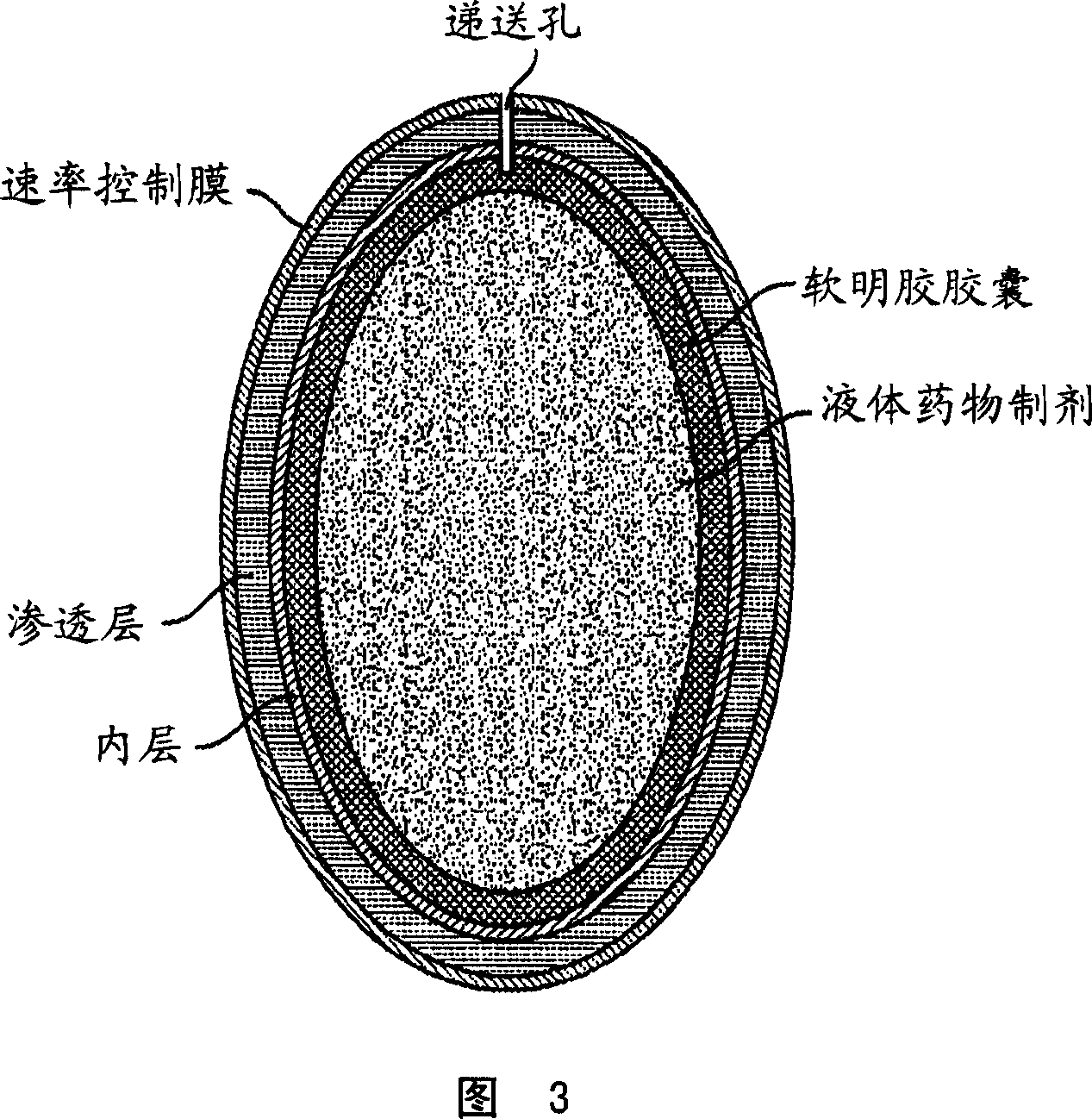
Methods of reducing alcohol-induced dose dumping for opioid sustained release oral dosage forms
A sustained-release, oral dosing technology, applied in pharmaceutical formulation, pill delivery, drug combination, etc., can solve problems such as respiratory weakness, opioid drug overdose, death, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0212] Example 1: Hydromorphone Tablet, Dual Layer 16mg System
[0213] The inventive sustained release dosage form of hydromorphone is adjusted, designed and shaped as an osmotic drug delivery body, which is prepared as follows: First, a pharmaceutical composition is prepared. 8.98 kg of hydromorphone hydrochloride, 2.2 kg of povidone (polyvinylpyrrolidone) known as K29-32 and 67.06 kg of polyethylene oxide with an average molecular weight of 200,000 were added to the basin of the fluid bed granulator. Then, 6.0 kg of povidone (polyvinylpyrrolidone) called K29-32 and having an average molecular weight of 40000 was dissolved in 54.0 kg of water to prepare a binder solution. The dry material was fluid bed granulated by spraying 18.0 kg of binder solution. Next, the wet granules are dried in a granulator to an acceptable water content and sized with a mill suitable for a 7-mesh screen. The granules were then passed to a mixer where they were mixed with 16 g of butylated hydrox...
Embodiment 2
[0218] Example 2: In Vitro Release Study - 16 mg Hydromorphone
[0219] A series of dissolution tests were performed with the hydromorphone tablets of Example 1 to evaluate the effect of alcohol on the in vitro release profile of a hydromorphone sustained release dosage form of the present invention containing 16 mg of hydromorphone (as hydromorphone hydrochloride). The release of hydromorphone hydrochloride was measured over 24 hours using a Type VII dissolution bath in aqueous solutions containing 0, 4, 20 and 40% by volume ethanol.
[0220] The hydromorphone hydrochloride 16 mg tablets of Example 1 were used to determine the release rate and cumulative release profile in 0%, 4%, 20% and 40% ethanol. Release rate results were applied to 0% ethanol (water) conditions from the 0 month stable time point. Additional samples from the 0-month stable extension point were used to generate release rates for 4%, 20% and 40% ethanol conditions. Release rate conditions are as follows:...
Embodiment 3
[0238] Embodiment 3: comparative study of release in vitro
[0239] As a comparison, Hydromorphone HCl release from Palladone XL(R) 32 mg capsules was assessed using a Type II dissolution bath in Vodka (27% v / v ethanol) and water to compare the hydromorphone tablets of Example 1 .
[0240] The dissolution parameters are as follows: dissolution equipment: Varian VK7010 dissolution unit and VK8000 autosampler; medium: water and vodka (Pavlova, 40% volume ethanol) respectively; volume: 900mL; stirring speed: 50rpm; suction volume: 5mL; temperature: 37±0.5°C; time points: T=1, 2, 4, 6, 10, 14, 18 and 24 hours. NOTE: Test results show that Pavlova has an alcohol concentration of only 27%.
[0241] Due to the color interference of vodka, the sample solution in vodka was evaporated before analysis as follows: Fill a 5 mL volume sample solution into a test tube with an autosampler. After cooling to room temperature, a volume of 2 mL of the sample solution was added to the scintilla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com