Penehyclidine quaternary ammonium salt and its derivative
A technology of penehyclidine and quaternary ammonium salt is applied in the field of anticholinergic compounds and their preparation, and can solve the problems of heart rate influence, inability to take long-term medication, senile dementia and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Methyl penehyclidine quaternary ammonium iodide salt
[0037] The preparation method of methyl penehyclidine quaternary ammonium iodide salt:
[0038] The raw material penehyclidine and excess CH 3 1, add in the reaction bottle, then add acetonitrile and make raw material dissolve, then stir, reflux reaction 7-10 days, thin-layer chromatography monitors until reaction reaches equilibrium; Cool to room temperature, distill off solvent, residue is cleaned with ethyl acetate 2 -3 times, the target compound was obtained by ethanol recrystallization, and the measured yield was more than 50%.
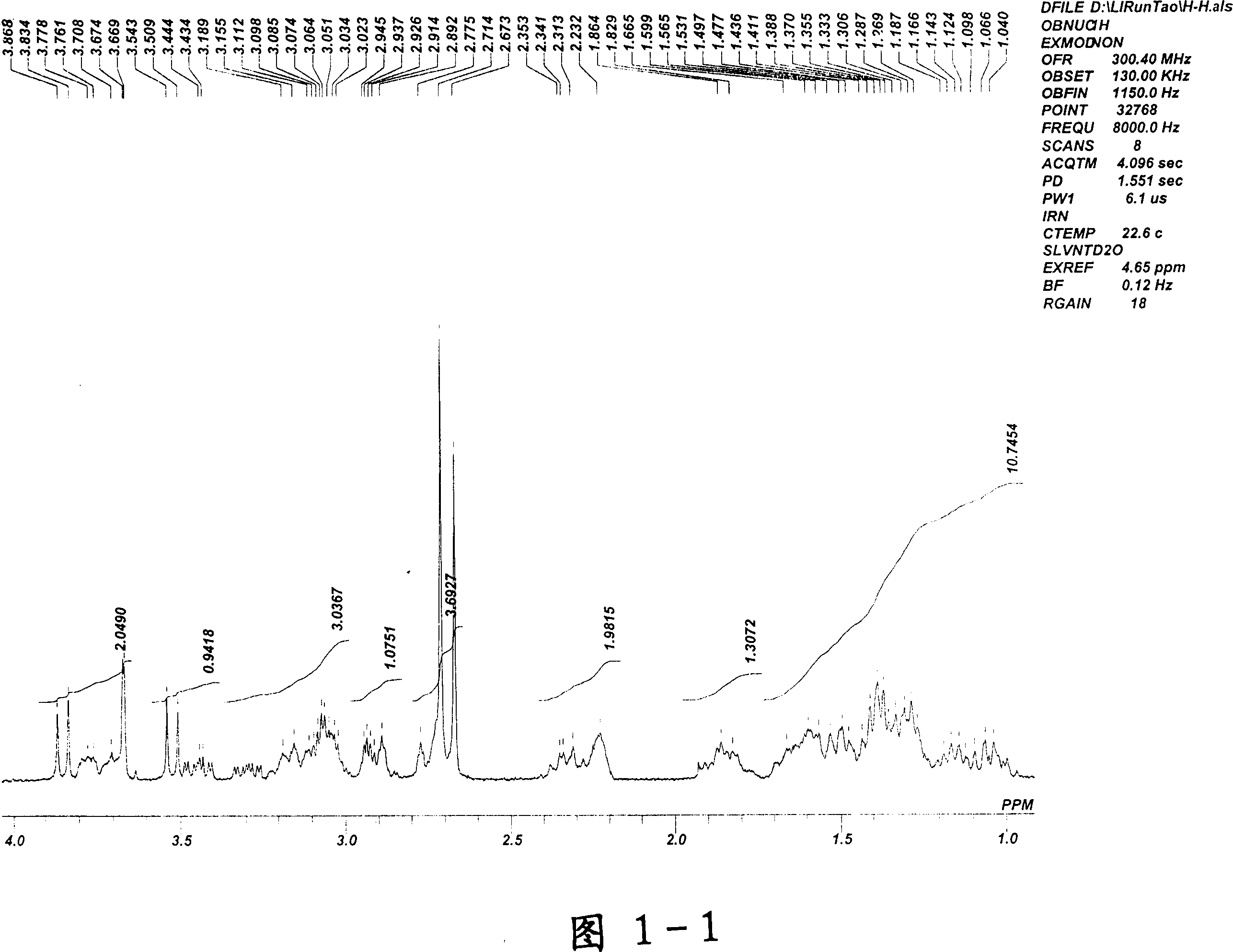
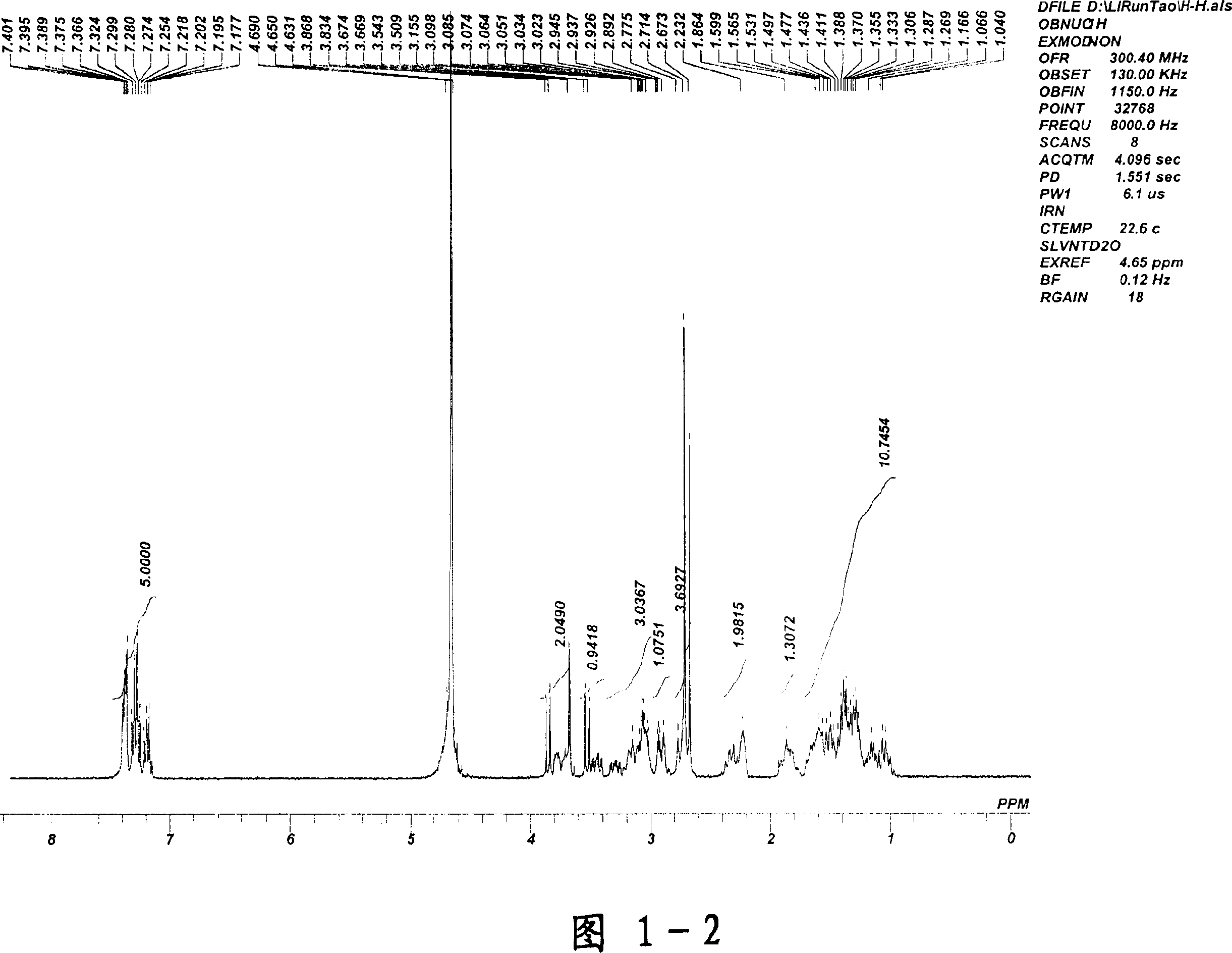
[0039] After proton nuclear magnetic resonance verification, see attached drawings 1-1 to 1-2 (continuous spectrum of proton nuclear magnetic resonance spectrum), this structure is methyl penehyclidine quaternary ammonium iodide salt.
[0040] Pharmacodynamic experiments have proved that methyl penehyclidine quaternary ammonium iodide salt cannot enter the blood-brain b...
Embodiment 2
[0041] Embodiment 2: Isopropyl penehyclidine quaternary ammonium iodide salt
[0042] The preparation method of isopropyl penehyclidine quaternary ammonium iodide:
[0043] The raw material penehyclidine and excess CH 3 CH I CH 3 , add in the reaction bottle, then add acetonitrile to dissolve the raw material, then stir, reflux for 3-7 days, monitor by thin-layer chromatography until the reaction reaches equilibrium; cool to room temperature, evaporate the solvent, and wash the residue with ethyl acetate for 2- The target compound was obtained by ethanol recrystallization three times, and the measured yield was more than 50%.
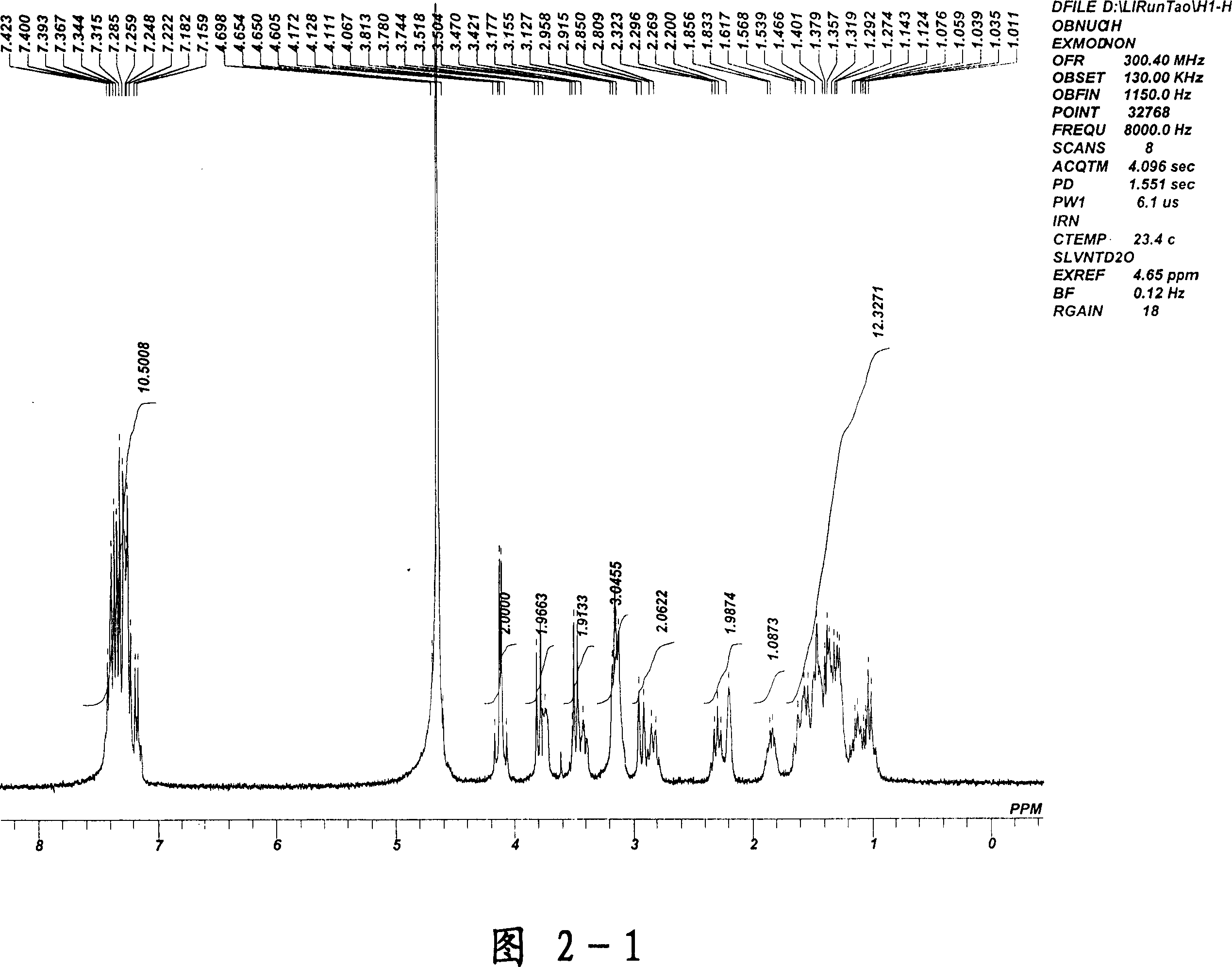
[0044] Verified by H NMR spectroscopy, the structure is isopropyl penehyclidine quaternary ammonium iodide salt.
[0045] Pharmacodynamic experiments have proved that penehyclidine quaternary ammonium iodide salt cannot enter the blood-brain barrier, has no anti-tremor effect, but has anti-salivation effect, has no effect on the central nervous system,...
Embodiment 3
[0046] Embodiment 3: Ethyl penehyclidine quaternary ammonium hydrochloride
[0047] The preparation method of ethyl penehyclidine quaternary ammonium hydrochloride:
[0048] The raw material penehyclidine and excess CH 3 CH 2 Cl, add in the reaction flask, then add acetonitrile to dissolve the raw material, then stir, reflux reaction for 5-7 days, thin-layer chromatography monitoring until the reaction reaches equilibrium; evaporate the solvent, and the residue is washed 2-3 times with ethyl acetate, The target compound was obtained by recrystallization from ethanol, and the measured yield was more than 50%.
[0049] Verified by H NMR spectrum, the structure is ethyl penehyclidine quaternary ammonium hydrochloride.
[0050] Pharmacodynamic experiments have proved that penehyclidine quaternary ammonium hydrochloride cannot enter the blood-brain barrier, has no anti-tremor effect, but has anti-salivation effect, has no effect on the central nervous system, and is well tolerat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com