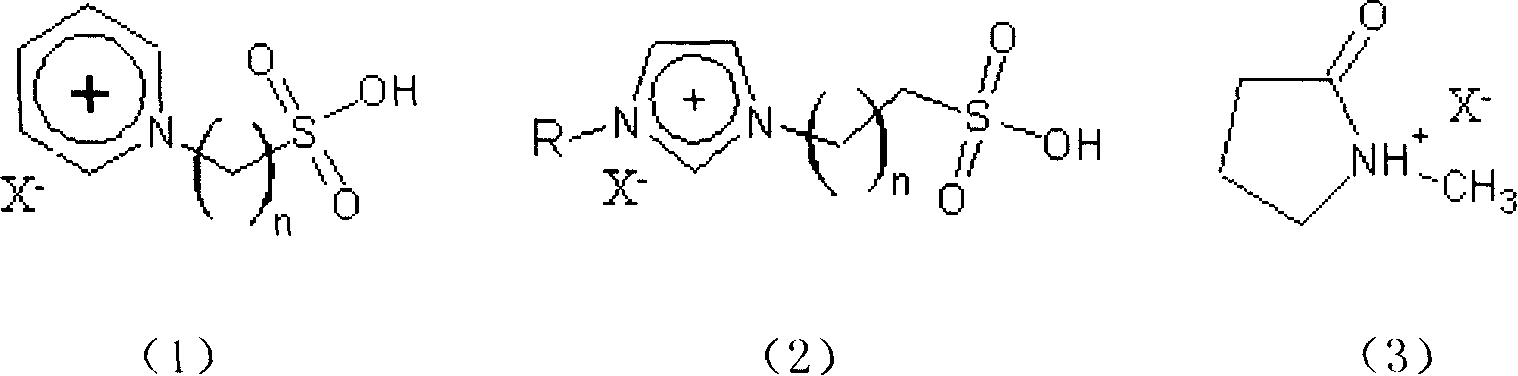
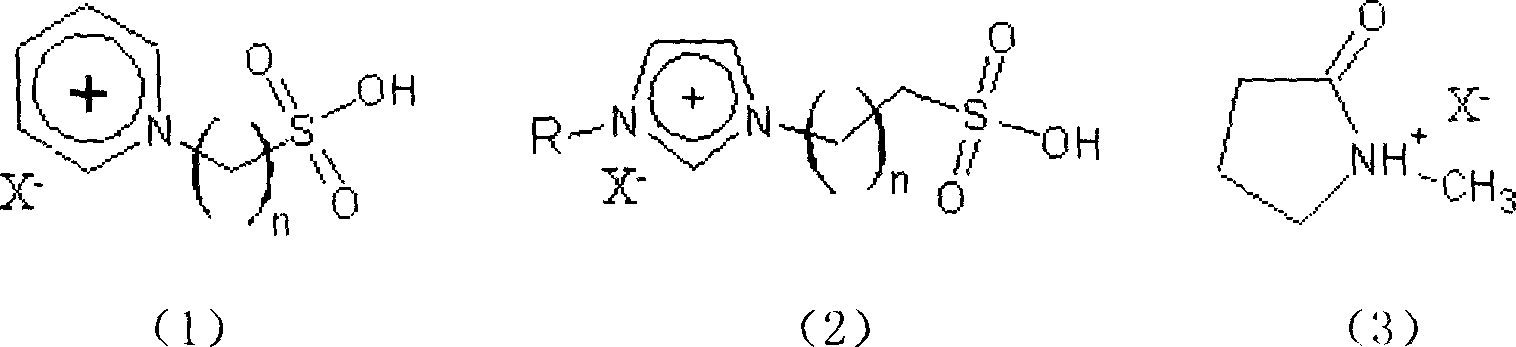Clean esterification method for producing bialkyl ortho phthalate
A dialkyl phthalate, esterification technology, applied in chemical instruments and methods, carboxylate preparation, organic chemistry and other directions, can solve the problems of difficult separation and recovery, equipment corrosion, low selectivity, etc., to achieve separation The effect of easy process, high esterification ability and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Put 6g of ionic liquid 1-methyl-3-(propyl-3-sulfonic acid)imidazolium fluoroborate, 5.7g of phthalic anhydride, 10g of isooctyl alcohol and an appropriate amount of water-carrying agent into the reaction flask, Stirring and heating, the esterification temperature is 110-125°C, and react for 1.5 hours. After the reaction solution is allowed to stand and separate into layers, the upper reaction solution is poured out and purified by distillation. The conversion rate of phthalic anhydride is 98.1%, and the selectivity is 98.6%.
Embodiment 2
[0024] Put 2g of ionic liquid 1-methyl-3-(propyl-3-sulfonic acid) imidazole hydrogen sulfate, 5.7g of phthalic anhydride, 12.5g of n-octanol and an appropriate amount of water-carrying agent into the reaction flask, Stirring and heating, the esterification temperature is 110-130°C, and react for 2 hours. After the reaction solution is allowed to stand and separate into layers, the upper reaction solution is poured out and purified by distillation. The conversion rate of phthalic anhydride is 97.2%, and the selectivity is 98.5%.
Embodiment 3
[0026] Put 20g of ionic liquid 1-methyl-3-(propyl-3-sulfonic acid)imidazole p-toluenesulfonate, 5.7g of phthalic anhydride, 15g of isooctyl alcohol and an appropriate amount of water-carrying agent into the reaction flask , stirred, heated, the esterification temperature was 100-125°C, and reacted for 2 hours. After the reaction solution was allowed to stand and separate, the upper reaction solution was poured out and purified by distillation. The conversion rate of phthalic anhydride was 98.2%, and the selectivity was 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com